It is rare to find hearts in which the great arterial trunks arise from their morphologically appropriate ventricles, but are abnormally related one to the other, with the aorta in an anterior position. As far as we are aware, such an abnormal arrangement of the ventriculo-arterial junctions was first reported by Theremin in 1895.1 In 1939, Harris and Farber2 suggested that the arrangement should be called “anatomically corrected transposition”. This was because, at that time, the accepted criterion for “transposition” was that the aorta was positioned anteriorly relative to the pulmonary trunk, irrespective of the ventricular origin of the arterial trunks. Since the essence of the unusual situation was the anterior location of the aorta (Fig. 1), the description was entirely logical for that era. The situation was deemed to be “anatomically corrected” because the arterial trunks arose from their morphologically appropriate ventricles. Most investigators, however, were dubious about the provenance of such anomalies, often arguing that the situation represented an “embryological impossibility”. Then, in 1967, Van Praagh and Van Praagh3 provided unequivocal evidence of the existence of the malformation. They described two patients, each with usual atrial arrangement and left juxtaposition of the right atrial appendage. In both cases, they showed the aorta arising in a left-sided and anterior position from the morphologically left ventricle. Having initially3 retained the term “transposition” when describing such hearts, Van Praagh4 subsequently suggested that the lesion should be called ‘anatomically corrected malposition', seeking to distinguish it from other hearts with “transposition”.4 He argued that, to be properly “transposed”, the arterial trunks should arise from morphologically inappropriate ventricles, albeit that not all experts, at that time, were convinced by his arguments.5 Irrespective of how the arrangement was to be described, several small series, and multiple single cases, have since been published,6–22 further attesting to the existence of this malformation. It remains a fact, nonetheless, that many paediatric cardiologists and surgeons remain unaware of the significance of the malformation, or else are unsure of its make-up, in particular its distinction from other malformations which are currently described using arcane terms such as “isolated ventricular inversion”,23–30 or “isolated ventricular discordance”.31, 32 These terms are not immediately meaningful, and it could be argued that the term “anatomically corrected malposition” would better be applied to the normal heart, in which any malpositions are certainly anatomically corrected. Irrespective of such niceties, the unifying feature of all these unusual entities is that the arterial trunks, whilst arising from their morphologically appropriate ventricles, exit from the ventricular mass in parallel rather than spiral fashion.33 We have recently encountered several such hearts within the pathological archive of the Hospital for Sick Children, Toronto.34 Seeking further to clarify their appreciation and understanding, we have also collected all examples of similar combinations that we could find thus far reported in the English language. Having analysed this collective experience, we show how categorization and description is greatly simplified when account is taken separately of the ventricular origin of the arterial trunks, the nature of the infundibular structures supporting the arterial valves, and the relationships of the intrapericardial arterial trunks. It remains to be determined how they are best described, but we would opt for the discarding of cryptic titles such as “anatomically corrected malposition”.
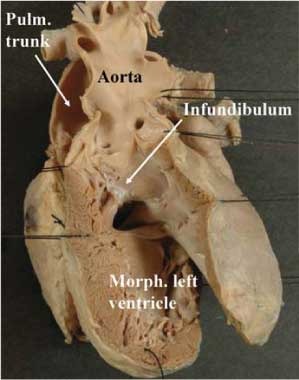
Figure 1. The illustration shows an anterior and left-sided aorta arising above a complete muscular infundibulum from the morphologically left ventricle. The atrioventricular connections are concordant in this heart, as are the ventriculo-arterial connections, despite the anterior location of the aorta and its support by a complete infundibulum. This is the essence of so-called “anatomically corrected malposition”. Note that the arterial trunks exit from the heart in parallel fashion.
Segmental analysis
As with all so-called “complex malformations”, the key to understanding the markedly heterogeneous anatomic variation to be discussed is to appreciate that description of the ventricular origin of the arterial trunks, the relationships of the trunks one to the other, and the nature of their infundibular support, accounts only for the arrangement of the arterial segment of the heart. As might be anticipated, and as confirmed by our review of the existing literature, these arrangements can be found with markedly different combinations of the other cardiac segments. Logical analysis, therefore, demands the use of a sequential segmental approach.35 It is now accepted that an integral part of such analysis is to identify the topological arrangement of components of the cardiac segments, specifically the morphologically right and left atriums, the morphologically right and left ventricles, and the arterial trunks, basing this analysis on their most consistent anatomic features. This is the essence of the so-called “morphological method”.36 As we will show, this principle is particularly important when considering the various lesions to be reviewed.
Use of the principle dictates that atrial morphology be determined according to the shape of the atrial appendages, specifically by the extent of the pectinate muscles within the vestibules of the atrial chambers. Thus, the morphologically right appendage is a broad-based triangle, its pectinate muscles extending all round the atrioventricular junction. In contrast, the morphologically left appendage has a narrow base and a tubular shape, with the pectinate muscles confined to the anterior margin of the vestibule. For the ventricles, it is the pattern of the apical trabeculations that is the defining characteristic. The morphologically right ventricle has coarse apical trabeculations, with the septal leaflet of its atrioventricular valve, when present, having multiple cordal attachments to the septum. The apical trabeculations of the morphologically left ventricle, in contrast, are finer, and the surface of the trabecular component of septum is smooth. The atrioventricular valve in the left ventricle hardly ever has septal cordal attachments. Analyzing the morphology of the chambers on the basis of their apical trabeculations permits all ventricles to be distinguished, even when they lack one or more of their components, and hence are incomplete. The ventricular mass, of course, extends from the atrioventricular to the ventriculo-arterial junctions. The infundibular structures, or ventricular outlets, therefore, are an integral part of the ventricular mass. In this respect, the morphologically right ventricle typically possesses a complete muscular tube supporting the leaflets of its arterial valve. As we will see, the morphologically left ventricle can also, on occasion, possess such a completely muscular infundibulum, while the muscular outflow tract can also be deficient in the morphologically right ventricle. The arterial trunks are recognized on the basis of their pattern of branching, with the aorta giving rise to the coronary and brachiocephalic arteries, and the pulmonary trunk bifurcating into the right and left pulmonary arteries.
Describing the ventriculo-arterial junctions
If we are to appreciate the nature of an abnormal relationship between the great arteries, we must of necessity understand first the features of normality. In the so-called “normal” relationship between the arterial trunks, with right hand ventricular topology, the aorta takes its origin from the base of the left ventricle in a right-sided and posterior position, wedged between the atrioventricular valves, with the pulmonary trunk swinging leftward from the anteriorly positioned morphologically right ventricle before dividing into the right and left pulmonary arteries (Fig. 2). In the much less common setting of left hand ventricular topology, the “normally related” aorta arises in a left-sided and posterior position, and the pulmonary trunk arises anteriorly from the right-sided infundibulum of the mirror-imaged right ventricle (Fig. 3). In both situations, the arterial trunks spiral round one another as they exit from the ventricular mass, with some of the leaflets of the aortic valve typically being in fibrous continuity with the aortic leaflet of the mitral valve, whereas the leaflets of the pulmonary valve are supported by the complete muscular infundibular tube, or conus, of the right ventricle (Fig. 4).

Figure 2. The cartoon shows the usual relationships of the arterial trunks when there is right hand ventricular topology. The pulmonary trunk spirals round the right-sided aorta, having exited from the base of the morphologically right ventricle.

Figure 3. When there is left hand ventricular topology, then the “normal” arrangement of the arterial trunks is also the mirror-image of the usual pattern (see Fig. 2).

Figure 4. This illustration shows the outflow tracts of the normal heart viewed from the ventricular apexes. Note the fibrous continuity between the leaflets of the aortic and mitral valves, and the complete muscular infundibulum which supports the leaflets of the pulmonary valve.
In so-called “anatomically corrected malposition”, when there is right hand ventricular topology, the aorta continues to take origin from the morphologically left ventricle, but in a left-sided and anterior position when assessed relative to the pulmonary trunk at their origins from the ventricular base (Fig. 1). When found in the setting of left hand ventricular topology, the aorta arises in right-sided and anterior position from the right-sided morphologically left ventricle (Fig. 5). In both situations, the arterial trunks then exit in a parallel fashion from the ventricular mass. In the majority of cases described and reported thus far in this fashion, the aortic valve arising concordantly from the morphologically left ventricle is supported by a complete muscular infundibulum (Fig. 6).

Figure 5. In this heart, there is double inlet left ventricle with left-sided incomplete right ventricle, indicative of left hand ventricular topology. The ventriculo-arterial connections, however, are concordant, albeit with parallel arrangement of the arterial trunks. This is an example of so-called “anatomically corrected malposition”. Abbreviation: AV: atrioventricular.

Figure 6. The close up of the outflow tract of the heart shown in Figure 5 reveals the presence of a complete muscular infundibulum supporting the leaflets of the aortic valve above the cavity of the dominant left ventricle. Abbreviation: RAVV, LAVV: right and left atrioventricular valves.
A very similar situation, however, has been described as “isolated ventricular inversion”. This term is applied to the segmental combination of usual atrial arrangement with discordant or double inlet atrioventricular connections, but with concordant ventriculo-arterial connections (Fig. 7). The aorta arises in a right-sided position, from the right-sided morphologically left ventricle, but with fibrous continuity between the leaflets of the aortic and one or both of the atrioventricular valves (Fig. 7). Yet another similar situation is described as “isolated ventricular non-inversion”. This arcane term accounts for the segmental combination of mirror-imaged atrial arrangement with discordant atrioventricular but concordant ventriculo-arterial connections. The aorta arises in left-sided position from the morphologically left ventricle, with fibrous continuity between the leaflets of the aortic and the atrioventricular valve or valves.

Figure 7. In this heart, the atrioventricular connections are discordant, but the ventriculo-arterial connections are concordant. There is fibrous continuity between the leaflets of the aortic and mitral valves in the roof of the right-sided morphologically left ventricle, and the arterial trunks exit from the heart in parallel fashion. Apart from the difference in infundibular morphology, and the presence of discordant rather than double inlet atrioventricular connections, the morphology of the outflow tracts is comparable to that seen in the heart shown in Figures 5 and 6.
In all of these settings, the unifying feature is that the arterial trunks arise from morphologically appropriate ventricles, and course in parallel fashion as they exit from the ventricular mass. It is the infundibular morphology, and the atrioventricular connections, that differ in the various subsets described with different names. As emphasized in the morphological method,36 however, it is inappropriate to define one variable feature on the basis of another anatomic feature that is itself variable. Using the concept of the morphological method,36 therefore, there is no justification for describing all these various entities with different names. In our opinion, it is much better to concentrate on the concordant ventricular origin of the parallel arterial trunks as the unifying diagnostic feature.
Review of the literature
When reviewing the English literature at the end of 2005, we found 72 cases with concordant ventriculo-arterial connections, but with a parallel rather than spiral arrangement of the arterial trunks. Of these cases, the majority (92%) had usual atrial arrangement, with 2 cases described with mirror-imaged atrial arrangement, 3 with isomeric atrial appendages, and one case with the arrangement of the atrial chambers not being specified. An abnormal position of the cardiac apex was a common finding amongst all the descriptions. These cases can then be sub-divided on the basis of variations at the atrioventricular junctions.
Usual atrial arrangement and concordant atrioventricular connections (Table 1)
As might be expected, usual atrial arrangement with concordant atrioventricular connections was the most common segmental combination, accounting for just under half (43%) of the descriptions. An abnormal position of the right atrial appendage37 was a frequent association, described in 39%. The ventricular septum was deficient in most hearts within the group, and an abnormal right atrioventricular junction, along with obstruction of the subpulmonary or subaortic outflow tracts, was a frequent association. In one case, the aorta was right-sided in the presence of segmental disharmony. In this particularly unusual heart, there was usual atrial arrangement, the atrioventricular connections were concordant, but there was left-handed ventricular topology.38 Within the overall group, the arrangement of the coronary arteries was not always described, but when this information was available, three-fifths had a solitary coronary artery. The majority also had complete muscular infudibulums supporting both arterial trunks, with the aorta being anterior and left sided.3, 7–11, 14, 18, 20, 34, 37 One case is described, nonetheless, with fibrous continuity between the aortic and mitral valves, with the aorta being posterior and left-sided.12 In another case, there was bilateral deficiency of the muscular infundibulums, with aortic-to-mitral as well as pulmonary-to-tricuspid valvar continuity.16
Table 1. Usual atrial arrangement with concordant atrioventricular connection.

Usual atrial arrangement with discordant atrioventricular connections (Table 2)
This combination is the second most common segmental arrangement, accounting for just over one quarter (26%) of cases. All cases again have deficient ventricular septation, but in this group the atrial appendages are typically normally positioned, but subaortic obstruction is frequent. In almost three-fifths of cases (58%), the aortic valve is posterior and right-sided, with a subpulmonary infundibulum lifting the pulmonary trunk away from the ventricular base.3, 23–24, 27, 31–33, 39 Rarely, the aorta was described as being anterior and right-sided, with either bilateral or subaortic infundibulums.22, 31
Table 2. Usual atrial arrangement with discordant atrioventricular connection.

Usual atrial arrangement with univentricular atrioventricular connection (Table 3)
Overall, just over one-fifth (22%) of reported cases exhibited univentricular atrioventricular connections. Of these, greater than four-fifths (81%) had absence of the right atrioventricular connection, with the left atrium communicating with a dominant left ventricle, the incomplete right ventricle being in right-sided and anterior position, in other words the typical findings of tricuspid atresia. The remaining one-fifth (19%) were described with double inlet to a morphologically left ventricle, with the incomplete right ventricle being anteriorly positioned and either right- or left-sided. Both juxtaposition of the right atrial appendage, and obstruction of the subpulmonary outflow tract, were frequent associations. The pattern of the coronary arteries was described in only a few patients, but when described was abnormal in all. All variants of infundibular morphology have also been described, but bilateral infundibulums are most frequent.
Table 3. Usual atrial arrangement with univentricular AV connections (dominant LV).

Mirror imagery and isomerism of the atrial appendage (Table 4)
Mirror imaged arrangement of the atrial appendages has been reported twice, with one patient having concordant atrioventricular connections with pulmonary atresia, ventricular septal defect and a single coronary artery.7 The second patient6 had discordant atrioventricular connections, with a ventricular septal defect and unobstructed outflow tracts. Bilateral infundibulums were present in both instances. In 1974, Freedom and Harrington13 described a patient having the appropriate arrangements at the ventriculo-arterial junction in the setting of asplenia, raising the possibility of the patient also having right isomerism, but detailed description of the morphology of the appendages was lacking. Crossland et al.40 have now described a patient with isomerism of the right atrial appendages, while in our archive of congenitally malformed hearts in Toronto, we found two cases with isomeric left atrial appendages,34 both being additionally juxtaposed.
Table 4. Mirror imagery and isomerism of the atrial appendage.

Discussion
As illustrated not only by our personal experience with autopsied specimens from the Toronto archive,34 but also from our review of the literature, patients with concordant ventriculo-arterial connections but parallel arrangement of the arterial trunks can be found with all arrangements of the atrial appendages, and with all varieties of atrioventricular connections.3, 6–34, 37–39, 41–46 As we have emphasized, sequential segmental analysis permits all these rare and complex malformations to be diagnosed and described in logical and simple fashion. The constant feature of the hearts reviewed is the parallel nature of the concordantly connected arterial trunks as they exit from the ventricular mass. In the past, subgroups of hearts having this feature in common have been described in arcane and variable fashion according to the specific infundibular morphology. But there is no constancy of infundibular morphology, even amongst the groups of hearts stratified in this fashion. Furthermore, the concept of naming hearts on the basis of an anatomical feature that is itself variable contravenes the “morphological method”, this being perhaps the most important principle for the description of congenitally malformed hearts developed over the latter part of the twentieth century.36
To emphasise again, therefore, all the hearts we have reviewed have in common the features of concordant ventriculo-arterial connections, but with parallel rather than spiral arrangement of the arterial trunks as they exit from the ventricular mass. It is these features that should serve as the basis of diagnosis. In our opinion, the hearts would best be described without recourse to the term “anatomically corrected malposition”. The hearts fulfilling the diagnostic criterions are exceedingly rare. The term “anatomically corrected malposition” is not immediately understandable, and if used, will create confusion relative to the other esoteric descriptor of “isolated ventricular inversion” and its variations. It is the parallel nature of the arterial trunks which will raise suspicion of the existence of the malformation. When such parallel arterial trunks are most frequently encountered in the setting of congenital cardiac disease, then the ventriculo-arterial connections are either discordant, or else both trunks arise from the same ventricle. The finding of parallel trunks, but with concordant ventriculo-arterial connections, will be sufficiently rare in most centres to warrant full segmental description, including careful analysis of infundibular morphology. The rareness of the lesion, and perhaps lack of awareness of its existence during life, is demonstrated by the fact that we did not discover any such cases coded thus far in the extensive echocardiographic records of the Hospital for Sick Children in Toronto, despite searching for such findings in patients with tricuspid atresia, and others with juxtaposition of the atrial appendages, both known to be harbingers of the combination of concordant ventriculo-arterial connections and parallel arterial trunks. Despite the absence of such cases in our records of living patients, we discovered 8 examples of the combination in our archive of congenitally malformed hearts,34 showing that the lesions are better recognized as pathological curiosities rather than on the basis of important features seen in life.
It is, of course, the fact that the ventriculo-arterial connections are concordant despite the abnormal infundibular morphology and arterial relationships that makes the lesions so unusual. When found in the setting of concordant atrioventricular connections, the circulations are potentially normal, albeit that all patients described thus far have had deficient ventricular septation. When the atrioventricular connections are discordant, in contrast, the patients present as if having physiologically uncorrected transposition, again always in the setting of deficient ventricular septation. Recognition of this latter arrangement is crucial, since these are the perfect patients for repair using atrial redirection, nowadays usually achieved using the Senning procedure. Knowledge of the location of the ventricular conduction tissues will then be essential for the surgeon when closing the co-existing ventricular septal defect, and relieving any associated interventricular malformations. To the best of our knowledge, the specific arrangement of the conduction tissues has yet to be established in these rare lesions. Since the aortic valve is most frequently supported by a muscular infundibulum, it is possible that the subaortic outflow tract does not interpose between the atrial and ventricular septal structures, so there could well be a regular atrioventricular node, or even a sling of conduction tissue around the ventricular septal defect. These features need to be clarified. In the setting of isomeric atrial appendages, the ventricular conduction tissues are determined by ventricular topology and the orientation of the ventricular septum.47 It is the position of the ventricular septum that dictates the location of the axis for ventricular conduction when the atrioventricular connections are univentricular. In these latter settings, therefore, the surgeon will be able to predict the location of the atrioventricular node and bundle, albeit that these are less troublesome in the current era of creation of extracardiac tunnels to produce the functionally univentricular circulation. It is the location of the sinus node that is more important, and this can be predicted with certainty by identifying the structure of the atrial appendages, albeit that there is no known position for the grossly hypoplastic atrial pacemaker found in the setting of isomeric left atrial appendages.
Certain of the associated malformations are sufficiently frequent to suggest morphogenetic linkages. Thus, abnormalities of the right atrioventricular junction suggest problems with expansion of the atrioventricular canal during development, and this also suggests a link with left juxtaposition of the atrial appendages. Of equal significance could be the frequent finding of malformations of the coronary arteries, particularly a solitary coronary artery. Although described rarely, when such information is available, then the majority of patients are known to have abnormalities in the disposition of the coronary arteries. This must be more than coincidence. Only with increasing diagnosis will the significance of these findings be known. This requires diagnosis on the basis of parallel arrangement of the concordantly connected arterial trunks, rather than depending on descriptions of cryptic lesions such as “anatomically corrected malposition” or “isolated ventricular inversion”.
Acknowledgements
Robert H. Anderson is supported by grants from the British Heart Foundation together with the Joseph Levy Foundation. Research at the Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust benefits from R&D funding received from the NHS Executive.












