Congenital heart defects afflicts approximately 0.8–1.2% of live births worldwide. Reference Liu, Chen, Zühlke, Black, Choy, Li and Keavney1–Reference van der Linde, Konings, Slager, Witsenburg, Helbing, Takkenberg and Roos-Hesselink3 A third of the defects can be expected to be severe and present as candidates for cardiac surgery. Reference Kim, Kim and Burt4,Reference Oster, Lee, Honein, Riehle-Colarusso, Shin and Correa5 Vocal cord palsy (VCP) is a recognised and important complication in children which can follow congenital cardiac surgery. VCP may result when either one or both of the recurrent laryngeal nerves are damaged; these children may present with speech, feeding, and respiratory impediments which are exaggerated in bilateral VCP. Reference Daya, Hosni, Bejar-Solar, Evans and Bailey6
Iatrogenic injury to the nerve fibres during an invasive surgical intervention may result in the paralysis of unilateral or bilateral vocal cords. Reference Raut, Maheshwari and Joshi7 Daya et al described that left-sided VCP was more common than bilateral VCP following paediatric cardiac surgery. Reference Daya, Hosni, Bejar-Solar, Evans and Bailey6
The risk of VCP occurring is contingent on patient and surgical aspects. Patient-related factors include female gender, pre-maturity, and low birth weight. Reference Rodney, Thompson, Anderson and Burkhart8,Reference Henry, Hsieh, Sanna, Vikse, Taterra and Tomaszewski9 Additionally, aortic arch manipulations and patent ductus arteriosus (PDA) ligations pose the most significant procedural risks of VCP. Reference Alfares, Hynes and Ansari10 Anticipating these factors better prepares the clinician for multidisciplinary collaboration – especially with otolaryngology and anaesthesiology for the management of emergency airway obstruction.
It is particularly important to consider the variable complications, long-term outcomes, and quality of life for the VCP patient. There will invariably be differences in the extent to which the nerves and vocal cords are affected between patients, and by extension, the degree to which phonatory and respiratory functions are affected. In an acute setting, airway obstruction is a life-threatening complication of VCP and if not treated can lead to aspiration, respiratory distress, and even death. Reference Alfares, Hynes and Ansari10 In the longer term, a developing child’s ability to engage in conversation or eat without the need of feeding tubes can all be compromised. This can also be detrimental to both the child and their family’s quality of life. Reference Karas, Patki, Ryan, Upchurch, Eapen and Raynor11 Appropriate knowledge on VCP is important as it can guide the diagnosis and management. Additionally, many areas of the multidisciplinary team such as cardiology, otolaryngology, anaesthesia, and speech-language therapy can benefit from this high-quality evidence. Furthermore, there are very few long-term follow-up studies that have assessed the quality of life and long-term impacts of children affected, making it difficult to dictate necessary clinical interventions following discharge.
The aim of this review is to summarise and evaluate the available evidence surrounding VCP following cardiac surgery in the paediatric population, with a broader focus on management strategies and the impact on quality of life for these patients.
Clinical assessment of patients with vocal cord palsy
The clinical presentation of a child with VCP can be split broadly into features affecting phonation and features affecting respiration. The most common, and sometimes the only feature of VCP, is the presence of stridor. Bilateral VCP has been reported to have a higher incidence and more severe presentation of stridor compared to unilateral VCP. Other symptoms include a weak cry, dysphagia, and potential aspiration. Intercurrent cardiac and neurological disorders can also complicate the clinical picture and cause or contribute to respiratory distress. Reference Daya, Hosni, Bejar-Solar, Evans and Bailey6
While the occurrence of VCP following cardiac surgery is rare, it is important to note that this is heavily reliant on the child’s clinical examination. As many patients may be asymptomatic or only present with subtle clinical signs after a certain period of time, they may have not been adequately investigated initially. Reference Alfares, Hynes and Ansari10 Investigations are either invasive or non-invasive, and each carry associated advantages and disadvantages to both the child and clinician.
Non-invasive methods
A non-invasive method that can be undertaken at the bedside is the use of ultrasound to study vocal cord mobility. Shaath et al Reference Shaath, Jijeh, Alkurdi, Ismail, Elbarbary and Kabbani12 conducted a study with 10 children who had undergone heart surgery and used ultrasound to assess the movements of the vocal cords. They found it was successful in recognising when VCP was not present. However, it was not as successful in diagnosing VCP as there could be some movement of the vocal cords despite being paralysed. Reference Shaath, Jijeh, Alkurdi, Ismail, Elbarbary and Kabbani12 Ultrasound is normally used as an adjunct to endoscopy rather than as an alternative.
Invasive methods
Laryngoscopy is an invasive intervention that is used for vocal cord visualisation. Literature suggests that fibreoptic flexible laryngoscopy should be utilised when investigating VCP and may be the only diagnostic option necessary as it allows clear visualisation of the vocal cord dynamics and the patency of the airway. Reference Handler13
Direct rigid laryngoscopy can be conducted under general anaesthesia. A competitive advantage over flexible laryngoscopy is that the arytenoids can be palpated to exclude cricoarytenoid joint fixation, which is another differential of VCP. Reference Daya, Hosni, Bejar-Solar, Evans and Bailey6 However, the palpation should be done cautiously to avoid iatrogenic injury which could lead to prolonged intubation. The use of either flexible laryngoscopy or rigid laryngoscopy should be carried out regularly to assess the status of the vocal cords and to monitor after treatment for improvement. Reference Benjamin, Smith, Cotten, Jaggers, Goldstein and Malcolm14
Laryngeal electromyography can also be utilised to assess recurrent laryngeal nerve function. Monitoring electrodes are attached to the thyroarytenoid muscles intraoperatively to look at the extent of immobility. A retrospective study by Scott et al Reference Scott, Chong, Randolph and Hartnick15 found that in 50% of patients, the results from laryngeal electromyography resulted in a change in management for the patient. Additionally, Maturo et al Reference Maturo, Braun, Brown, Chong, Kerschner and Hartnick16 built on Scott et al’s Reference Scott, Chong, Randolph and Hartnick15 work and used laryngeal electromyography as a method to predict if recurrent laryngeal nerve function would return. In children who had undergone PDA ligation, they found that the absence of action potentials by 6 months meant it was unlikely that the VCP would resolve. Reference Maturo, Braun, Brown, Chong, Kerschner and Hartnick16
Having expressed that routine diagnosis ought to replace symptomatic diagnosis, the utility of diagnostic methods must be considered. While flexible laryngoscopy is the preferred choice, it can be challenging to perform in younger children. Nonetheless, it performs better than direct laryngoscopy, which requires a greater level of user expertise, and in smaller neonates, it may require general anaesthesia. Non-invasive methods such as ultrasound are also very user-dependent and may not be opted for in severe or life-threatening presentations of VCP. The diagnostic method is crucial in the assessment and treatment of VCP not only does it dictate the next step but can also be used to predict the outcome for the child and hence the quality of life. Regular use of flexible laryngoscopy is recommended to assess the patency of the airway and vocal cord status.
Management
Management options for VCP can be split into two options: medical and surgical.
Following diagnosis, the clinician will be able to evaluate the need, type, and urgency of treatment.
Medical management
Before deciding the modality of treatment, the child can be observed through clinical observation and serially measuring oxygen saturations to check for respiratory distress. Reference Daya, Hosni, Bejar-Solar, Evans and Bailey6 If the child shows signs of airway compromise, more urgent intervention is required. Spontaneous recovery is an option for VCP in stable children where a “wait and watch” method is adopted. The mechanism of this process is not well understood; however, in unilateral VCP, it is hypothesised that the healthy vocal cord can compensate for the other until neurological regeneration occurs. Reference Setlur and Hartnick17 During this time, the use of a nasogastric tube is common until the child is able to adequately cough and swallow. Reference Dewan, Cephus, Owczarzak and Ocampo18
Another option is speech therapy which has been found to be successful, particularly in older children. While there is a lack of reported evidence for VCP following cardiac surgery, 84% of patients with neurological malignancies found improvement in both speech and swallowing function. Further clinical trials are necessary to ascertain if this remains true for children with VCP following cardiac surgery.
Steroid therapy is favoured due to its likelihood of helping to reduce post-operative voice changes by preventing surgery-related complications such as oedema, which are linked to voice change. Reference Lachanas, Exarchos and Tsiouvaka19 Aside from their analgesic, prophylactic, and antiemetic properties, steroids are also able to promote post-paresis nerve function recovery Reference Misono and Merati20 and are used as part of the treatment of hoarseness. Reference Espinosa and Ongkasuwan21 A single dose of intraoperative steroids can reduce the duration of temporary recurrent laryngeal nerve palsy; however, it does not reduce the rate of recurrent laryngeal nerve palsy Reference Misono and Merati20 nor does it have a beneficial effect on voice-related quality of life. Reference Lachanas, Exarchos and Tsiouvaka19
Surgical management
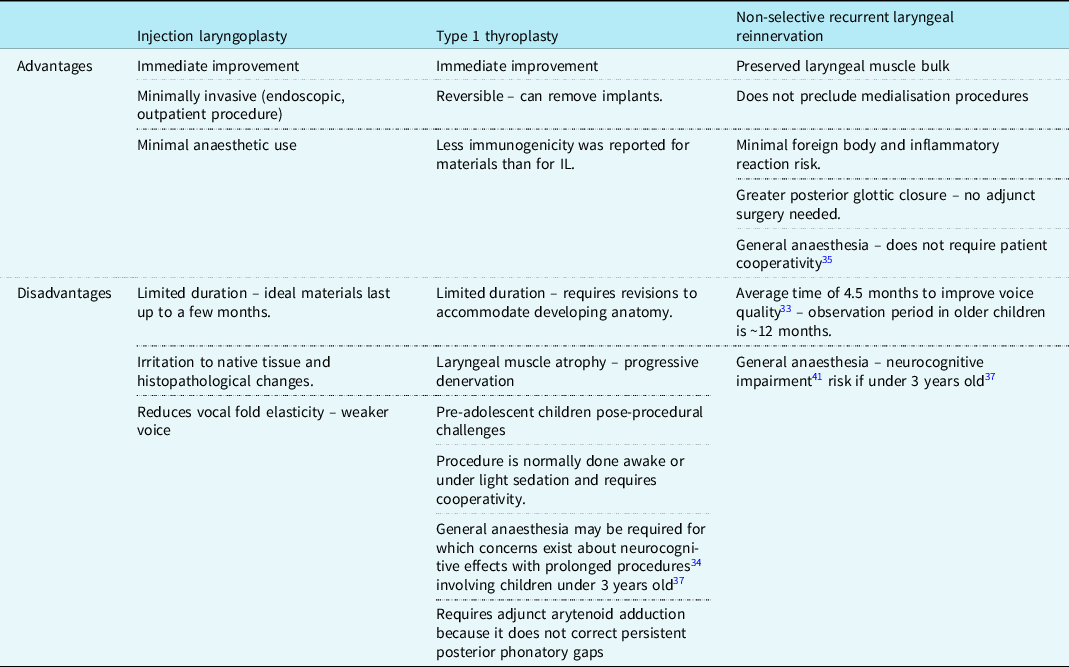
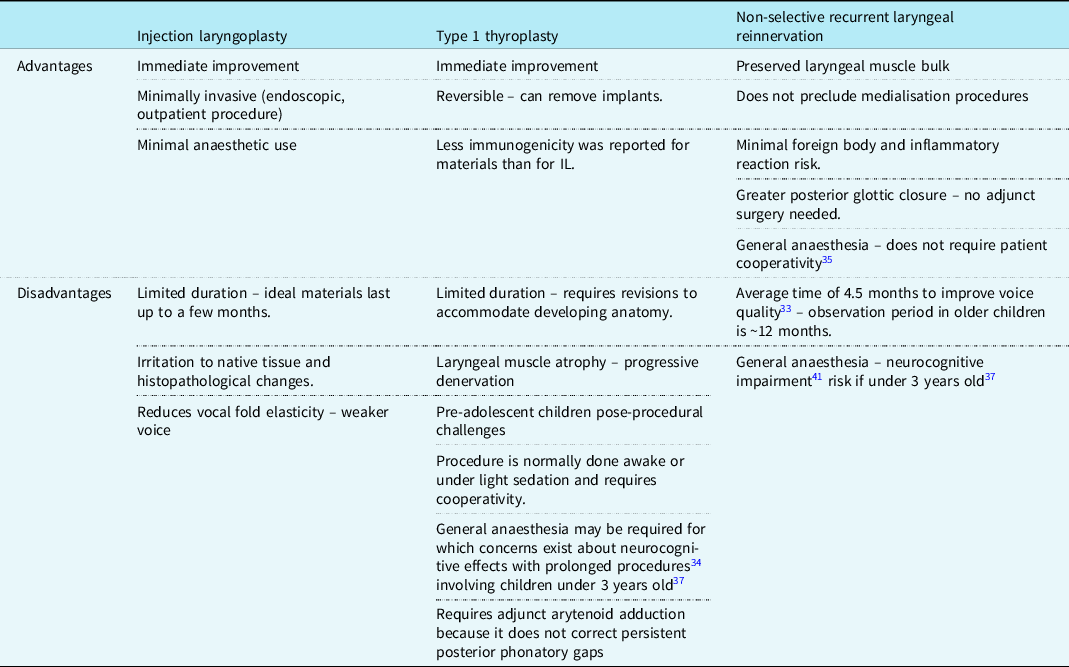
Surgical management is undertaken after the recommended 8–12 months period of observation, unless the child is initially unstable and requires early surgical intervention to protect the airway. Findings from Jabbour et al Reference Jabbour, Martin, Beste and Robey22 and Misono et al Reference Misono and Merati20 indicate that between 20 and 40% of children remain symptomatic with unilateral VCP and emerge as candidates for surgical intervention. The main surgical interventions for unilateral VCP are medialisation (injection laryngoplasty or type 1 thyroplasty) and non-selective recurrent laryngeal innervation and as shown in Table 1, each procedure carries its own set of advantages and disadvantages. Reference Espinosa and Ongkasuwan21 The most popular bilateral VCP interventions are tracheostomy and iterations of posterior cordotomy. Reference Anthony, Parker, Patel and Halum23
Table 1. Comparison of the positives and drawbacks of injection laryngoplasty, type 1 thyroplasty, and NSLR adapted from Espinosa et al Reference Espinosa and Ongkasuwan21 unless indicated otherwise
Surgical management for unilateral vocal cord palsy
Injection laryngoplasty
This involves injecting a synthetic material into the paralysed cord to medialise it. Reference Espinosa and Ongkasuwan21 The aim is to allow phonation and prevent aspiration, as a temporising intervention either to mitigate symptoms or in anticipation of spontaneous reinnervation Reference Zur and Carroll24 ; it is also used in the immediate post-operative period prior to non-selective laryngeal nerve innervations. Reference Espinosa and Ongkasuwan21 It has great utility in treatment plans aiming to initiate and/or advance an oral diet in children with surgically induced VCP, especially if conducted within 6 months of the surgical injury. Reference Meister, Johnson and Sidell25 There are a variety of materials available with variable immunogenicity such as Teflon, hyaluronic acid, collagen, Reference Espinosa and Ongkasuwan21 and carboxymethylcellulose, which despite lasting between 1 and 2 months, it is a strong material option as it has a safe biological profile with few reported complications and good outcomes in voice improvement. Reference Espinosa and Ongkasuwan21
Type 1 thyroplasty
Type 1 thyroplasty is a technically demanding and invasive procedure involving the insertion of synthetic implants through a window fashioned in the thyroid cartilage. The materials used are either Silastic, cartilage, or Gore-Tex, Reference Misono and Merati20,Reference Zeitels, Mauri and Dailey26 for which there are less reported immunogenic effects than for injection laryngoplasty materials. Reference Maturo, Braun, Brown, Chong, Kerschner and Hartnick16 Butskiy et al Reference Butskiy, Mistry and Chadha27 reported 88% (n = 8) improvement or recovery from aspiration in children, and this finding could be the substrate of further clinical study amongst larger cohorts. Revisions are necessary, however, in children to accommodate the growing and developing laryngeal anatomy. Reference Espinosa and Ongkasuwan21 The anticipated amount and frequency of these revisions are difficult to deduce from the scarcity of available long-term follow-up data. Reference Butskiy, Mistry and Chadha27 In any case, delayed access is understood to cause increasingly greater technical challenges, especially if conducted post-puberty. Reference Maragos28 Owing to its failure to correct persistent phonatory gaps, Reference Espinosa and Ongkasuwan21 Isshiki et al Reference Isshiki, Tanabe and Sawada29 first described arytenoid adduction as a relatively simple yet critical adjunct to type 1 thyroplasty, despite its leading to longer procedural times and being technically challenging. Reference Hoffman, Surender, Chapin, Witt, Mcculloch and Jiang30
Reinnervation
Non-selective recurrent laryngeal reinnervation of the abductor and adductor intrinsic muscles of the larynx is achieved by anastomosing the recurrent laryngeal nerve to itself or an adjacent motor nerve. Reference Anthony, Parker, Patel and Halum23 In comparison to static medialisation thyroplasty, non-selective recurrent laryngeal reinnervation confers a higher voice quality due to better vibrational capacity of the vocal cords Reference Marie, Hansen, Brami, Marronnier and Bon-Mardion31,Reference Paniello, Edgar, Kallogjeri and Piccirillo32 with more long-term stability owing to preserved laryngeal muscle bulk and tone. Reference Marie, Hansen, Brami, Marronnier and Bon-Mardion31 It confers a significantly reduced risk of aspiration in children due to the improved glottic closure patterns. Reference Zur and Carroll24 It has been recommended that the optimal time for non-selective recurrent laryngeal reinnervation is after 3 years of age. Reference Espinosa and Ongkasuwan21 Although general anaesthesia Reference Smith, Roy and Houtz33 obviates the need for intraoperative patient cooperativity, there are concerns related to the neurocognitive harm caused by prolonged anaesthetic time. Reference Ing, DiMaggio and Whitehouse34
The independence from synthetic materials allows laryngeal development and removes the necessity for revision surgeries that are associated with thyroplasty. The procedure is understood to be safe without a significant risk of airway or wound complications, Reference Smith, Roy and Houtz33,Reference Sipp, Kerschner, Braune and Hartnick35 but a consultation with a paediatric cardiologist would be useful in any case when assessing operative risk. Unlike the medialisation techniques, the improvement seen with unilateral VCP is not immediate. Findings from Smith et al Reference Smith, Roy and Houtz33 – which shows consistency with other studies from a systematic review of laryngeal reinnervation techniques Reference Sipp, Kerschner, Braune and Hartnick35 – suggest that a positive improvement from reinnervation can be expected after a minimum of 6 months with stable results by 12 months. Having already undergone previous cardiac surgery, patients (and/or their parents) may show resistance to the prospect of further surgery due to additional scarring Reference Zur and Carroll24 and so ought to be counselled appropriately on the implications and long-term benefits. Ongkasuwan et al Reference Ongkasuwan, Espinosa, Hollas, Devore, Procter, Bassett and Schwabe36 suggests that NSLR procedures should be performed as early as possible to optimise outcomes but also noted that favourable outcomes could still be achieved two decades after the onset of childhood neuronal VCP. They also speculate that pre-operative laryngeal electromyography may have utility in predicting voice outcomes following NSLR but that further data are required. Reference Ongkasuwan, Espinosa, Hollas, Devore, Procter, Bassett and Schwabe36
Surgical management for bilateral vocal cord palsy
Iterations of posterior cordotomy
This is an irreversible procedure that improves the laryngeal airway by removing a posterior section of the vocal fold, while potentially sacrificing voice and/or swallowing ability. Reference Anthony, Parker, Patel and Halum23 Despite modification by otolaryngologists in recent years to mitigate these compromises, the treatment approach remains suboptimal. Reference Li, Garrett and Zealear37
Tracheostomy
A tracheostomy involves either a temporary or permanent opening of the trachea. This gives relief of airway obstruction and protection from aspiration but does not improve phonation (and will remove any ability to phonate unless fenestrated). Reference Trozzi, Meucci, Salvati, Tropiano, Bottero and Hart38 Despite a tracheostomy improving the airway, Reference Anthony, Parker, Patel and Halum23 findings by Westwood et al Reference Westwood, Hutchins and Thevasagayam39 substantiate that there is an inherent compromise on quality of life for both the patient and their caregivers and recommend psychosocial support for families. The main problem is the stoma that requires continual care, Reference Young and Rosen40 and so research of less-invasive techniques is ongoing. Reference Trozzi, Meucci, Salvati, Tropiano, Bottero and Hart38
Selective reinnervation
In contrast to non-selective recurrent laryngeal reinnervation, selective recurrent laryngeal innervation of the posterior cricoarytenoid muscles involves specifically reinnervating distal abductor branches of the recurrent laryngeal nerve. Reference Anthony, Parker, Patel and Halum23 Crumley Reference Crumley41 was instrumental in defining the phrenic nerve as a donor target for the abductor muscle bellies. A case series by Lee et al Reference Lee, Bon-Mardion, Smith and Marie42 enforced a belief of the technique’s safety and appropriate use in children as young as 2 years old. Evidence remains largely limited to adult patients however, and further studies with larger sample sizes are warranted for this promising technique.
Arytenoidectomy
This is the irreversible surgical removal of the arytenoid cartilage to enlarge the airway. Reference Hoffman, Surender, Chapin, Witt, Mcculloch and Jiang30 Advances in arytenoidectomy procedures started with the use of endoscopic lasers, which resulted in increased precision and decreased oedema post-surgery, and most importantly, did not require tracheostomy to be performed. Reference Trozzi, Meucci, Salvati, Tropiano, Bottero and Hart38 However, surrounding tissues can be damaged by the heat from the lasers, leading to increased susceptibility to granuloma and scar formation, and thus resulting in more revision surgeries as the airways will inevitably re-narrow. Reference Zeitels, Hochman and Hillman43 Arytenoidectomy can be combined with arytenoid cordectomy or used alone, yet both procedures can adversely affect voice quality. Reference Li, Garrett and Zealear37
Laterofixation (Suture lateralisation/laterofixation)
Laterofixation of the vocal cord and/or arytenoid cartilage is indicated in bilateral VCP patients as an alternative to tracheostomy or as a temporising measure when the recovery of laryngeal function is expected. Reference Damrose44 It reversibly enlarges the airway without damaging phonatory tissues, so that voice can be later be restored and it can be performed independently or concurrently to endoscopic procedures. Reference Li, Garrett and Zealear37 Good success rates have been reported in paediatric cohorts Reference Damrose44 with superior performances in aerodynamic measures when compared to irreversible static procedures (e.g., cordotomy and arytenoidectomy); nonetheless, complications including aspiration, hoarseness, and need for adjustments should be anticipated. Reference Li, Garrett and Zealear37
Laryngeal pacing (Functional electrical stimulation)
Laryngeal pacing is an upcoming surgical option that has the potential to confer high-quality ventilatory improvement in the absence of swallowing and phonatory compromise. Reference Li, Garrett and Zealear37 As demonstrated by Mueller, Reference Mueller and Pototschnig45 the laryngeal pacing system involves electrode stimulation of the posterior cricoarytenoid muscle(s) with the implant being situated in a subcutaneous pocket of the sternum. Candidates with aberrant recurrent laryngeal nerve reinnervation will optimise the utility of this procedure. Reference Mueller and Pototschnig45 Future research ought to address the absence of paediatric data for this technique. Reference Trozzi, Meucci, Salvati, Tropiano, Bottero and Hart38 The actual procedure itself is complicated and also proves expensive as the device needs replacement at least every 10 years. Reference Shiotani, Saito, Araki, Moro and Watabe46
Botulinum injection
The toxin botulinum is also used in the treatment of bilateral VCP, especially in patients with laryngeal synkinesis, which is the involuntary movement of muscles following voluntary movement in one muscle. It promotes ventilation by causing flaccid paralysis in adductor muscles through inhibiting acetylcholine from being released from axon terminals. Reference Li, Garrett and Zealear37 Once adductor inspiratory motor neurons and muscles are blocked, abductor inspiratory motor neurons are able to produce a glottal opening, thereby enabling ventilation. Unlike cordotomy and arytenoidectomy, this treatment option has little to no effect on the voice. However, it is only a temporary fix as repeated injections are required every 3 months. Reference Li, Garrett and Zealear37
Future management options
Further research could offer enhanced detail into our understanding of VCP and allow better diagnosis and treatment. Alternative therapy that promise regeneration of the laryngeal nerves and muscle trophism is gene therapy. Reference Li, Garrett and Zealear37 Gene therapy offers exciting prospects; while untested in the paediatric community, gene transduction is in pre-clinical trials for adult patients. Vectors can be delivered to certain points of the body such as the nervous system, musculature, or mucosa around the larynx. This could provide protection to the neurons, regeneration of the axons, and also prevention of muscle degeneration. Reference Araki, Suzuki, Uno, Tomifuji and Shiotani47 However, while gene therapy is under consideration for the treatment of bilateral VCP caused by neurodegenerative conditions, it does not prevent synkinesis. Reference Li, Garrett and Zealear37 Gene therapies also pose a risk of neuronal damage via the delivery of viral vectors into the CNS. Reference Trozzi, Meucci, Salvati, Tropiano, Bottero and Hart38 Gene therapy is still in the experimental stages, and more research is needed to test the effectiveness of neuronal regeneration and to find solutions to their adverse effects, before it can be considered as treatments for iatrogenic bilateral VCP, especially in the paediatric population.
Outcomes
The outcome of patients with VCP post-cardiac surgery varies with respect to a number of factors such as site (either unilateral or bilateral), gestational age, and surgical procedure. The complications following VCP can either be transient or permanent and vary widely in severity. Reference Benjamin, Smith, Cotten, Jaggers, Goldstein and Malcolm14
Recovery
A recent systematic review by Engeseth et al Reference Engeseth, Olsen, Maeland, Halvorsen, Goode and Røksund48 reported low left VCP recovery rates (0–33%) from a range of PDA ligation studies (17 retrospective; 4 prospective) which exposes infants to lifelong complications. Table 2 supports that the recovery of vocal fold function following congenital cardiac surgery is subject to substantial variation based on surgical procedure and gestational age. The highest rate of recovery is seen with aortic arch repair, specifically the Non-Norwood procedures with 86%, while the lowest rate of recovery is seen in PDA ligation with 0%. Reference Pourmoghadam, DeCampli, Ruzmetov, Kosko, Kishawi, O’Brien, Cowden, Piggott and Fakioglu49,Reference Fan, Campbell, Clarke, Washington, Fix and White50 Supportive care should be implemented during the post-operative period to aid the recovery of VCP. Reference Alfares, Hynes and Ansari10 It is also important to observe that a variation exists in the length of follow-up periods for VCP recovery across different studies which range between 3 and 16.4 months in Table 2. Many studies have relatively short follow-up periods which may prompt underestimated VCP recovery rates – there could be under detected greater rates of resolution within asymptomatic cohorts. Reference Fan, Campbell, Clarke, Washington, Fix and White50 Additionally, without conducting pre- and post-operative flexible laryngoscopy, there are inconsistencies in the evaluation of vocal cord motion before and after therapeutic intervention which challenges the comparability of studies.
Table 2. A summary on the incidence, recovery, and follow-up period for VCP following cardiac surgery Reference Alfares, Hynes and Ansari10,Reference Karas, Patki, Ryan, Upchurch, Eapen and Raynor11,Reference Jabbour, Martin, Beste and Robey22,Reference Pourmoghadam, DeCampli, Ruzmetov, Kosko, Kishawi, O’Brien, Cowden, Piggott and Fakioglu49–Reference Zbar, Chen, Behrendt, Bell and Smith52,Reference Pereira, Webb, Blakely, Cox and Lally56,Reference Khariwala, Lee and Koltai60

Truong et al Reference Fan, Campbell, Clarke, Washington, Fix and White50 observed that pre-mature infants, especially neonates (≤28 weeks gestational age), had lower rates of recovery of vocal cord function in comparison to infants born at term across a variety of cardiac surgeries. Evidence from Zbar et al Reference Zbar, Chen, Behrendt, Bell and Smith52 suggests that infants of greater pre-maturity and lower birth weight are more likely to develop left VCP during PDA ligations. Birth weight also appears to play a focal role in the incidence of left-sided VCP following PDA ligation; a greater incidence was found in infants with a lower birth weight (5 of 22; 22.7%), compared to the incidence for heavier babies (1 of 46; 2.2%). Reference Zbar, Chen, Behrendt, Bell and Smith52 Infants that weigh ≤1 kg at birth are defined as extremely low birth weight, and it is preferential to use clips during PDA ligation in this population to lessen the extent of dissection and mitigate the risk of injury to the great vessels. However, using clips is associated with higher rates of VCP; it can result in recurrent laryngeal nerve injury due to the close proximity of the recurrent laryngeal nerve to the ligation site. Reference Zbar, Chen, Behrendt, Bell and Smith52 Meticulous monitoring of extremely low birth weight infants undergoing PDA ligation is imperative – Benjamin et al Reference Benjamin, Smith, Cotten, Jaggers, Goldstein and Malcolm14 found an increased likelihood of bronchopulmonary dysplasia, reactive airway disease, or insertion of a gastrostomy tube in this cohort.
Adverse outcomes
Engeseth et al Reference Engeseth, Olsen, Maeland, Halvorsen, Goode and Røksund48 highlighted that left VCP is associated with a range of adverse outcomes among infants. There is a wide range of adverse outcomes spanning respiratory outcomes and comorbidities such as bronchopulmonary dysplasia, laryngeal outcomes (dysphonia, laryngomalacia, and subglottic stenosis), feeding outcomes (gastrostomy and tube feeding), and other outcomes (hospital stay and readmission rate). Reference Engeseth, Olsen, Maeland, Halvorsen, Goode and Røksund48 For example, a retrospective study by Nichols et al Reference Nichols, Jabbour and Hehir53 reviewed the functional outcomes with vocal fold immobility in patients after isolated PDA ligation. The presence of symptoms such as dysphonia (48%), dysphagia (27%), enteral tube feeds (24%), and respiratory symptoms, that is , stridor and increased work of breathing (11%) were observed in this patient cohort with a median follow-up of 3 years. This paper concluded that there is an association between the severity of clinical dysfunction at presentation and clinical outcomes. Reference Nichols, Jabbour and Hehir53
Karas et al Reference Karas, Patki, Ryan, Upchurch, Eapen and Raynor11 advocate that parents and primary care providers should be counselled that there is a 46% probability of children requiring a surgical feeding tube after sustaining VCP post-cardiac surgery. They also discuss, in addition to others, Reference Skinner, Halstead, Rubinstein, Atz, Andrews and Bradley54–Reference Pereira, Webb, Blakely, Cox and Lally56 that infants undergoing correction of larger structural defects with longer operative times and more extensive dissection near the recurrent laryngeal nerve have shown greater rates of intubations which is speculated to be due to lesser contralateral vocal fold compensation. Reference Setlur and Hartnick17
Quality of life
Considering the patient’s quality of life is crucial to better understand the consequences of VCP and its treatment. Reference Haraldstad, Wahl and Andenæs57 Despite a catalogue of available instruments for its assessment, quality of life remains a complex concept that is defined and interpreted in a variety of ways across and within various medical disciplines globally. Reference Haraldstad, Wahl and Andenæs57 The utility of the Paediatric Voice-Related Quality of Life (pVRQOL) survey was validated in a study by Boseley et al Reference Boseley, Cunningham, Volk and Hartnick58 as a more comprehensive instrument than the Paediatric Voice Outcomes Survey (pVOS). While pVRQOL results report social-emotional and physical-functional effects, pVOS is less sensitive and only documents global pre-operative and post-operative changes. Reference Boseley, Cunningham, Volk and Hartnick58 Walz et al Reference Walz, Hubbell and Elmaraghy59 found that the pVRQOL score in pre-mature children with a history of cardiac surgery is significantly lower than those without a history of cardiac surgery. There was no explanation given as to whether the reason for a decrease in the pVRQOL score was due to recurrent laryngeal nerve injury. To our knowledge, there is no literature that directly associates quality of life, recurrent laryngeal nerve injury (and hence VCP), and paediatric cardiac surgery. All references to quality of life are thus noted to be anecdotal in nature.
Post-cardiac surgery, it is clear that children are at significant risk of aspiration (which could lead to pulmonary injury) and dysphagia due to vocal fold dysfunction. Reference Zbar, Chen, Behrendt, Bell and Smith52,Reference Skinner, Halstead, Rubinstein, Atz, Andrews and Bradley54,Reference Khariwala, Lee and Koltai60 Reports documenting post-operative occurrences are abundant, yet there is a scarcity of data describing long-term follow-up. Reference Engeseth, Olsen, Maeland, Halvorsen, Goode and Røksund48,Reference Truong, Messner, Kerschner, Scholes, Wong-Dominguez, Milczuk and Yoon51,Reference Richter, Ongkasuwan and Ocampo61 The effects of dysphonia can be devastating for the social development of a child resulting in shyness, withdrawal at school, and general difficulty in engaging with others. Reference Rodney, Thompson, Anderson and Burkhart8
Rodney et al Reference Rodney, Thompson, Anderson and Burkhart8 – whose comparative analysis showed non-significant differences across the majority of post-operative variables between VCP and non-VCP neonates except for aspiration – suggested that the quality of life is predominantly impacted when there is insufficient self-resolution of the VCP or when a diagnosis is missed. Follow-up with an otolaryngologist is therefore of major importance yet may be overlooked due to lack of fail-safe institutional measures to ensure that every VCP patient receives a follow-up appointment. This could also be amplified further by insufficient foresight from the cardiac team to refer to otolaryngology when noting VCP during their designated follow-up. Reference Rodney, Thompson, Anderson and Burkhart8 Retrospective findings from Jabbour et al Reference Jabbour, Martin, Beste and Robey22 revealed only a 24% resolution rate (n = 278) in their paediatric cardiac surgery population – which is similar to the 35% (n = 80) reported by Truong et al. Reference Truong, Messner, Kerschner, Scholes, Wong-Dominguez, Milczuk and Yoon51 They also proposed a regimented follow-up plan that could be adopted for all paediatric VCP patients as shown in Figure 1. Reference Jabbour, Martin, Beste and Robey22

Figure 1. A flow chart summarising the recommended otolaryngology follow-up algorithm adapted from Jabbour et al. Reference Jabbour, Martin, Beste and Robey22
Importantly, Rodney et al Reference Rodney, Thompson, Anderson and Burkhart8 highlights that through effective counselling, physicians can fully prepare parents of aortic arch reconstruction patients to both manage aspiration with modified feeding and anticipate potential sequelae. This can also subdue concerns of aspiration-related pneumonia, gastrostomy, and tracheostomy (for bilateral VCP) due to their relative rarity. Reference Rodney, Thompson, Anderson and Burkhart8 Resiliency of the neonatal lungs was cited as a potential explanation for the evasion of respiratory sequelae. Reference Rodney, Thompson, Anderson and Burkhart8
As described by Dewan et al, Reference Dewan, Cephus, Owczarzak and Ocampo18 in addition to modified feeding, presentations of aspiration and dysphagia may require further dietary augmentation through delivery via gastrostomy or nasogastric tube. They found that VCP neonates are more likely to experience an abnormal cough and gag reflex and require an increased length of hospitalisation due to the tube feeding. Reference Dewan, Cephus, Owczarzak and Ocampo18 Supportive findings by Sachdeva et al Reference Sachdeva, Hussain, Moss, Schmitz, Ray, Imamura and Jaquiss55 indicated that 87% (n = 38) of VCP paediatric in their cohort were intolerant of full oral feeds at discharge and requiring modification to their nutritional intake, potentially with a modified delivery method. Recent findings from Richter et al Reference Richter, Ongkasuwan and Ocampo61 suggest that there is an expected time to regular oral feeding of less than 2 years irrespective of the infants’ VCP status and genetic comorbidities. Despite the good prognosis, parents of VCP infants should be informed of modified diets at discharge, and that there is a greater long-term risk for inpatient hospitalisation due to feeding difficulties and poor weight gain. Reference Richter, Ongkasuwan and Ocampo61
Sequelae of VCP such as prolonged tube feeding and dysphonia could significantly impact both the parent and the child as well as their relationship. More quality-of-life evaluations could be conducted in the future pertaining to the children and their families; voice-related quality of life, for instance, is a metric that could be better represented. While Walz. et al Reference Walz, Hubbell and Elmaraghy59 discussed voice-related quality of life for a pre-mature paediatric cohort, there could be further pVOS/pVRQOL short-term and long-term studies in a broader-aged paediatric cardiac surgery population for patients experiencing VCP. For older children, retrospective quality of life studies could be conducted to quantify the extent to which the condition affected their lives prior to intervention and be the substrate for additional future interdisciplinary interventions to better support this group.
Summary
Advances in paediatric cardiac surgery and medical care have caused a paradigm shift in patient care and survival. Patients with pre-maturity and complex congenital cardiac diseases are increasingly surviving their initial challenges and leading productive lives. The possibility of an increased prevalence of VCP in children is therefore likely, particularly with emerging undetected VCP cases. Variation in the reported causes of VCP is likely institution-specific and attributable to the paediatric cardiac surgery activity (Figure 2) Reference Daya, Hosni, Bejar-Solar, Evans and Bailey6,Reference Gorantla, Chan, Shen, Wilkes and Bratton63 – it is therefore difficult to ascertain fully representative statistics in regions where healthcare infrastructure remains underdeveloped.

Figure 2. A risk-stratification matrix depicting the various forms of cardiac paediatric surgery its’ subsequent risk on development of VCP based on the work by Gorantla et al. Reference Gorantla, Chan, Shen, Wilkes and Bratton63 CPB: cardio-pulmonary bypass; ASD: atrial septal defect; VSD: ventricular septal defect.
Patients at greater risk of VCP are female, pre-mature, and low birth weight undergoing either or both PDA ligation or aortic arch interventions. Avoiding the serious complications of aspiration and dysphagia is at the forefront of management decisions for the child – surgical feeding tubes are therefore common. Reference Karas, Patki, Ryan, Upchurch, Eapen and Raynor11 Clinicians should be prepared to counsel either or both patients and their families on the quality of life implications, which remains an area that requires further study. Certain events, such as aspiration-related pneumonia and gastrostomy, could be informed as rarities for aortic reconstruction patients. Reference Rodney, Thompson, Anderson and Burkhart8 On the contrary, clinicians should expect respiratory complications and enteral feeding for surgical PDA ligation patients. Reference Benjamin, Smith, Cotten, Jaggers, Goldstein and Malcolm14
Prospective multi-centre cohort studies are warranted in future and should aim to identify further VCP risk factors and better assess presentations such as voice and swallowing. Longer-term stricter institutional follow-ups will lend to enhanced understanding of true recovery rates. Pre- and post-operative flexible laryngoscopy should be conducted universally to prevent VCP underestimation in asymptomatic patients and promote early detection. Nonetheless, primary care providers should remain cautious as to not to overlook insidious cases of VCP which many present with similar symptoms to asthma in older children. Reference Røksund, Clemm, Heimdal, Aukland, Sandvik, Markestad and Halvorsen62
Conclusion
Cardiac surgery remains the leading cause of iatrogenic VCP in the paediatric population. Early recognition and management of VCP following cardiac surgery is vital to improve recovery and reduce morbidity and mortality. This is particularly important given that VCP can present insidiously or be mistaken for other conditions such as asthma. There are limited studies looking at VCP post-cardiac surgery, making it difficult to accurately assess and evaluate its implications, and translate findings into clinical practice. Follow-up studies targeting this cohort are warranted.
Acknowledgements
None.
Declarations
Nothing to declare.
Financial support
None.