Tetralogy of Fallot is the most common cyanotic CHD, with a survival rate of ~70%, 40 years after surgical repair.Reference Bertranou, Blackstone, Hazelrig, Turner and Kirklin 1 , Reference Cuypers, Menting and Konings 2 Although surgery improves prognosis, it is not a curative procedure, leaves residual lesions, and alters ventricular structure and dynamics.Reference Pillutla, Shetty and Foster 3 Postoperative pulmonary regurgitation is common and has an adverse impact on right ventricle haemodynamics, leading to eventual ventricular dilation and systolic dysfunction.Reference Frigiola, Redington, Cullen and Vogel 4 Right ventricular dysfunction confers a dismal long-term prognosis in patients with repaired tetralogy of Fallot, with reduced exercise capacity and increased risk of ventricular arrhythmias and sudden death.Reference Marie, Marçon and Brunotte 5 , Reference Friedberg, Fernandes and Roche 6 Therefore, right ventricular dilatation and systolic dysfunction are criteria for pulmonary valve intervention for significant pulmonary valve regurgitation.Reference Baumgartner and Bonhoeffer 7
Cardiac MRI is currently the gold standard to assess right ventricular volumes and ejection fraction,Reference Kilner, Geva, Kaemmerer, Trindade, Schwitter and Webb 8 useful in selecting the optimal timing for pulmonary valve replacement.Reference Knauth, Gauvreau and Powell 9 Although it is an invaluable imaging method to assess patients with tetralogy of Fallot who were previously repaired, it is more time consuming than transthoracic echocardiography, impeding its use for repeated evaluations.Reference Bernard, Morel, Descotes-Genon, Jehl, Meneveau and Schiele 10 In our centre, routine imaging follow-up is usually performed by echocardiography, and MRI is used when echocardiographic parameters or clinical status suggest that surgical re-intervention is to be considered; however, currently available echocardiographic parameters of right ventricular function have limited accuracy.Reference Rudski, Lai and Afilalo 11
Left ventricular global longitudinal strain has been proven to have a good correlation with left ventricular ejection fractionReference Benyounes, Lang and Soulat-Dufour 12 and to be a better prognostic marker than ejection fraction in a variety of cardiac diseases including chronic heart failure.Reference Nahum, Bensaid and Dussault 13 Right ventricular longitudinal strain has been hypothesised as an independent predictor of mortality in patients with pulmonary hypertension, with promising results.Reference Fine, Chen and Bastiansen 14 Nonetheless, little is known about its role in patients with repaired tetralogy of Fallot.
Therefore, we aimed to assess how right ventricular longitudinal strain correlates with right ventricular function indices, particularly ejection fraction, and functional parameters in patients with repaired tetralogy of Fallot.
Materials and methods
Patients with repaired tetralogy of Fallot followed-up in the grown-up CHD unit of our centre between 1975 and 2001 were retrospectively analysed. We excluded patients re-operated for pulmonary valve regurgitation and patients with residual right ventricular outflow tract obstruction. Only patients evaluated by 12-lead electrocardiogram, transthoracic echocardiography, MRI, and treadmill exercise testing, within a maximum time span of 12 months, were included.
Baseline clinical characteristics were collected from the medical records, including age, sex, weight, cardiovascular risk factors, medical therapy, surgical procedure, time since surgery, NYHA functional class, and follow-up time.
12-lead electrocardiogram
From the 12-lead electrocardiogram (25 mm/s for 1 mV), QRS width and the presence of right bundle brunch block pattern were assessed. Right ventricular strain – overload – pattern was defined as the presence of right bundle brunch block and positive T-waves in leads V1 and V2.Reference Luna 15
Transthoracic echocardiography
A complete transthoracic echocardiography was performed in all patients using Vivid-E9 (GE Healthcare Technology, General Electric Vingmed Ultrasound, Horten, Norway) device, with a 3-MHz probe. All examinations were performed by physicians specifically trained and experienced in imaging CHD, and subsequent offline analysis was performed by the same examiner, according to international recommendations.Reference Lang, Badano and Mor-Avi 16 Standard echocardiographic parameters were calculated; in addition, classic views – parasternal, apical, and subcostal – were adjusted to better assess the right ventricle. Right ventricular diameters were calculated in the parasternal long-axis and four-chamber views. The tricuspid annular systolic, excursion, right ventricular fractional area change, and tricuspid peak systolic velocity of tissue Doppler imaging (tricuspid S′) were assessed in the four-chamber view. The Tei index, or the global performance index, was calculated as the ratio between isovolumetric times – contraction and relaxation – and ejection time, using pulsed Doppler. Pulmonary regurgitation severity assessment included calculation of color Doppler jet length and area, vena contrata, and pressure half-time. Right ventricular longitudinal strain was analysed offline using the acquired cine loops in the four-chamber view with centered right ventricle (EchoPAC Program; GE Healthcare); these were stored with at least three cardiac cycles with a frame rate of 50–80 images/s. Speckle tracking analysis was performed using a validated software for the left ventricle. Endocardial borders were identified by the software and were manually corrected, and myocardial motion was automatically tracked. The right ventricle was divided into six segments: basal, medial, and apical from the septal and lateral walls. Longitudinal strain was determined as the average of all segments (Fig 1). In cases where it was not possible to analyse more than one segment per patient, strain data were not considered.
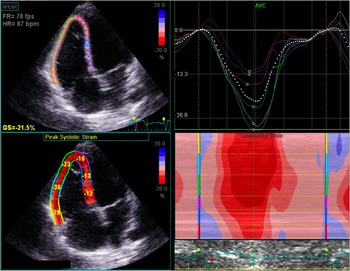
Figure 1 Right ventricular longitudinal strain assessment by transthoracic echocardiography. Up left: right ventricle-centred, four-chamber view with tracking of the myocardium; down left: strain value for each right ventricular segment; up right: strain curve of the right ventricular segments during the cardiac cycle.
Cardiac MRI
All patients were evaluated with cardiac MRI (Signa 1.5-T; GE Healthcare) to assess right and left ventricular volumes and ejection fraction, as well as pulmonary regurgitant volume and fraction.
Right and left ventricular volumes were measured by electrocardiographically triggered, breath-held, cine, steady-state, free-precession imaging sequences, according to the literature.Reference Kawel-Boehm, Maceira and Valsangiacomo-Buechel 17 The right ventricular volumes were obtained using short-axis slices from base to apex covering the entire length of the right and left ventricles; the endocardial contours were manually traced at end systole and end diastole. Assuming there may be a slight delay in end systole of the right ventricle in comparison with the left ventricle, especially in patients with right bundle branch block, the smallest right ventricular size was used to measure the right ventricular end-systolic volume. The pulmonary regurgitation volume was calculated using phase-velocity sequences, and pulmonary regurgitation was estimated using the ratio of regurgitant flow to total systolic forward flow.
Treadmill exercise test
Treadmill symptom-limited exercise testing was performed using the Bruce protocol in a Vmax Encore System equipment. In some patients, cardiopulmonary exercise testing with ventilatory expired gas analysis was also performed, using the modified Bruce protocol. In such cases, peak oxygen consumption and minute ventilation–carbon dioxide production relationship were analysed, in addition to the standard parameters.Reference Guazzi, Adams and Conraads 18
Statistical analysis
Baseline demographic and clinical data are described as mean±standard deviation for continuous variables and as frequencies and percentages for categorical variables.
Normality (Gaussian distribution) was tested for all continuous variables using the Shapiro–Wilk test. Right ventricular longitudinal strain and right ventricular ejection fraction showed a normal distribution (post-hoc). To assess the association between longitudinal strain and continuous variables, Pearson’s or Spearman’s correlation was used for normally or non-normally distributed variables, respectively. For categorical variables, comparison of longitudinal strain values between groups was assessed using the t-student test or the Mann–Whitney test for normally or non-normally distributed variables, respectively. To evaluate a potential independent association between right ventricular longitudinal strain and ejection fraction, the association between right ventricular function indices and ejection fraction was assessed by univariate – using the same methodology – and multivariate linear regression. Collinearity analysis between parameters was tested earlier. The best cut-off value of right ventricular longitudinal strain for predicting a right ventricular ejection fraction under 40%, which is often considered for surgical repair,Reference Warnes, Williams and Bashore 19 was identified using the receiver operating curve characteristic analysis, as the value with the best combined sensitivity and specificity. The level of significance considered was α=0.05. Data were analysed using Statistical Package for the Social Science for Windows, version 20.0 (SPSS Inc, Chicago, Illinois, United States of America).
Results
Baseline characteristics
Of the 42 patients included, 61% were male, with a mean age of 32±8 years, and all patients were in NYHA class I or II. The mean time since surgery was 27±5 years, and 65% had a transannular patch in the right ventricular outflow tract (Table 1).
Table 1 Baseline characteristics.

FAC=fractional area change; LV=left ventricle; LVEF=left ventricular ejection fraction; METS=metabolic equivalents; NYHA=New York Heart Association; RBBB=right bundle branch block; RV=right ventricle; RVEF=right ventricular ejection fraction; TAPSE=tricuspid annular plane systolic excursion; Tricuspid S′=systolic S-wave amplitude on the tricuspid annulus; VE/VCO2 slope=minute ventilation–carbon dioxide production relationship slope; VO2=oxygen consumption
Almost all patients (95%) showed a right bundle branch block in the 12-lead electrocardiogram (mean QRS width 145±33 ms), and 4 of them (10%) had this pattern with positive T waves in leads V1–V2 (Fig 2). Echocardiographic, MRI, and treadmill exercise testing data are detailed in Table 1.

Figure 2 Patient’s 12-lead electrocardiogram displaying right bundle branch block with positive T-wave in leads V1 and V2.
The mean longitudinal strain of the right ventricule was −16.2±3.7%. Strain data from at least five of the six segments were obtained in all patients, with a mean of 5.5 segments analysed per patient. The mean right ventricular ejection fraction was 43±7%, and the left ventricular ejection fraction was 58±8%, both determined by MRI; the mean pulmonary regurgitation fraction was 45±18%. Cardiopulmonary exercise testing was performed in 22 (53%) patients.
Association between right ventricular longitudinal strain and other right ventricular function parameters and functional indices
Repaired tetralogy of Fallot patients presenting an electrocardiogaphic pattern with right bundle branch block and positive T-waves in the V1–V2 leads had significantly lower right ventricular longitudinal strain (−10.9±2.8 versus −16.4±2.7%, p=0.025). Among echocardiographic parameters, the longitudinal strain showed significant linear correlation with tricuspid annular systolic excursion (r=−0.42, p=0.044) and a non-significant trend for association with pulmonary regurgitation severity assessed by colour length (r=−0.42, p=0.063). Longitudinal strain was not associated with other echocardiographic right ventricular function indices such as fractional area change or the Tei index. Longitudinal strain showed a linear correlation with right ventricular ejection fraction assessed by MRI (r=−0.45, p=0.019) (Fig 3). There was no significant correlation between right ventricular longitudinal strain and pulmonary regurgitation volume. There was also a trend for association between longitudinal strain and the metabolic equivalents achieved in treadmill exercise testing (r−0.45, p=0.054).
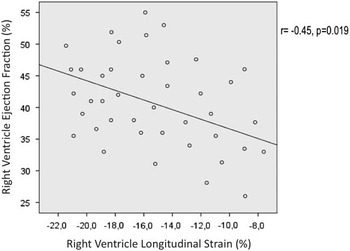
Figure 3 Correlation between right ventricular ejection fraction measured by cardiac MRI and right ventricular longitudinal strain assessed by speckle tracking analysis.
Predictors of right ventricular ejection fraction
In addition to right ventricular longitudinal strain, right ventricular ejection fraction showed a linear correlation with right ventricular fractional area change (r=0.50, p=0.004), colour length of pulmonary regurgitation (r=0.35, p=0.043), end-systolic volume (r=−0.60, p=0.001), and left ventricular ejection fraction (r=0.36, p=0.01). The presence of a patch in the outflow tract of the right ventricle was associated with a significantly lower right ventricular ejection fraction (43.8±7.9 versus 48.6±7.4%, p=0.014).
By multivariate analysis, longitudinal strain (β=−0.72, 95% confidence interval −1.41, −0.15, p=0.006) and left ventricular ejection fraction (β=0.39, 95% confidence interval 0.11, 0.67, p=0.008) were independently associated with right ventricular ejection fraction. Longitudinal strain had good discriminative power for predicting right ventricular ejection fraction <40%, with an area under the curve of 0.80 (95% confidence interval 0.59, 0.99, p=0.026) (Fig 4). A longitudinal strain of −17.0% was the best cut-off value for identifying a right ventricular ejection fraction <40%, with 80% sensitivity and 70% specificity.
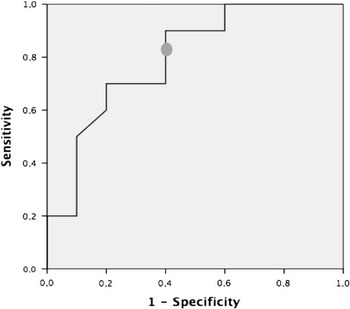
Figure 4 Receiver operating characteristics curve of the right ventricular longitudinal strain for predicting a right ventricular ejection fraction <40%. The cut-off value of −17.0% had 80% sensitivity and 70% specificity (area under the curve 0.80, 95% confidence interval 0.59, 0.99, p=0.029).
Discussion
Recently, 2D speckle tracking has emerged as an easy and reproducible tool to assess myocardial deformation. It allows the assessment of left ventricular systolic function, as it is less load dependent than ejection fraction.Reference Amundsen, Helle-Valle and Edvardsen 20 Moreover, it has proven to be a strong predictor of cardiovascular events.Reference Cho, Marwick, Kim, Kim, Hong and Oh 21 , Reference Stanton, Leano and Marwick 22 Analogously, right ventricular speckle tracking analysis has recently been applied to assess right ventricular function in patients with pulmonary hypertension, pulmonary embolism, and atrial septal defects. Reasonable correlation between longitudinal strain and right ventricular ejection fraction measured by MRI has been shown as well.Reference Jategaonkar, Scholtz, Butz, Bogunovic, Faber and Horstkotte 23 – Reference Pirat, McCulloch and Zoghbi 25 Nevertheless, scarce evidence supports the use of 2D speckle tracking longitudinal strain in repaired tetralogy of Fallot patients.
Right ventricular dysfunction and dilation are the main determinants of long-term outcome in these patients, associated with reduced exercise tolerance, quality of life, and increased risk of arrhythmias,Reference Wald, Haber, Wald, Valente, Powell and Geva 26 – Reference Gatzoulis, Balaji and Webber 28 and are critical determinants of surgical intervention for pulmonary valve disease. Nevertheless, the best echocardiographic parameter to assess right ventricular function is currently unknown.
Right ventricular contractility and stroke volume are mostly determined by longitudinal shortening of deeper muscle fibres inserted in the tricuspid annulus and in the right ventricular apex. Therefore, echocardiographic indices such as tricuspid annular systolic excursion and tricuspid S′ have been validated against right ventricular ejection fraction measured by MRI and proposed as prognostic relevant parameters.Reference Ramos, Branco and Agapito 29 Koestenberger et al, reported a positive correlation between tricuspid annular systolic excursion and right ventricular ejection fraction in children and adolescents with tetralogy of Fallot after surgical repair.Reference Koestenberger, Ravekes and Everett 30 Nonetheless, these parameters do not account for multisegmentar contractility. Many other parameters have been studied, including fractional area change, which accounts for different segments of the right ventricle, and the global myocardial performance index or the Tei index, but none has emerged as the best parameter to assess right ventricular function. Right ventricular ejection fraction estimation by 3D echocardiography is being evaluated, but very few data are available in previously operated patients with tetralogy of Fallot, and there is considerable intraobserver and interobserver variability,Reference Khoo, Young, Occleshaw, Cowan, Zeng and Gentles 31 mandating more data before routine clinical use.
Longitudinal strain is an easy tool, with low intraobserver and interobserver variability, and it accounts for the majority of segments responsible for right ventricular contraction ejection in a less load-dependent manner than ejection fraction.Reference Friedberg, Fernandes and Roche 6 In these patients with pulmonary regurgitation, the right ventricle is often enlarged and its curvature is no more longitudinal but rather circumferential. Nonetheless, strain assessment is an angle-independent technique. We evaluated the longitudinal strain of six segments of the right ventricle, including the interventricular septum. The assessment of right ventricle free-wall strain alone has been previously suggested;Reference Fukuda, Tanaka and Sugiyama 32 however, we believe that the interventricular septum should be included in the region of interest as it also contributes to right ventricular ejection and accounts for interventricular interaction, which plays a major role in right and left ventricular dysfunction in repaired tetralogy of Fallot patients, as previously described.Reference Geva, Sandweiss, Gauvreau, Lock and Powell 33 , Reference Piazza, Chessa and Giamberti 34 The right and left ventricular ejection fractions were correlated in our cohort. As the closure of the ventricular septal defect alters septal dynamics, including strain values of the septum may increase the prognostic value of right ventricular longitudinal strain. A few data are available on right ventricular longitudinal strain in repaired tetralogy of Fallot patients. Li et al, studied different 2D speckle tracking-derived parameters in these patients and reported a significant reduction in longitudinal strain in children and adults after surgery compared with a sex- and age-matched control group.Reference Li, Xie and Wang 35
Our study results revealed a significant association between right ventricular longitudinal strain and right ventricular ejection fraction by MRI. It was the only echocardiographic parameter that correlated with right ventricular ejection fraction in the multivariate analysis. This finding might have resulted from the fact that both parameters are derived from measurements acquired at end systole.Reference Lang, Badano and Mor-Avi 16 Longitudinal strain had good accuracy for predicting right ventricular dysfunction (area under curve 0.80), and an absolute value of longitudinal strain less than 17.0% was associated with 80% sensitivity and 70% specificity for predicting right ventricular ejection fraction under 40%, reinforcing the appropriateness of this method for routine evaluation of right ventricular function. Furthermore, longitudinal strain also had a trend for association with pulmonary regurgitation severity, which may reflect a higher contractile stress in a state of volume overload. Longitudinal strain also correlated with metabolic equivalents achieved during treadmill exercise testing. Nonetheless, longitudinal strain was not associated with maximal oxygen consumption, a strong marker of functional status, probably because of the small subset of repaired tetralogy of Fallot patients who also performed cardiopulmonary exercise testing. Of note, the right ventricular longitudinal strain was also associated with left ventricular ejection fraction, reinforcing the ventricular interdependence in these patients. Interestingly, patients presenting an electrocardiographic pattern with right bundle branch block and inappropriate T wave concordance in V1–V2, a marker of ischaemia or right ventricle pressure or volume overload, also had more severe dysfunction assessed by longitudinal strain.Reference Luna 15
In conclusion, evaluation of right ventricular longitudinal strain should be considered in the routine echocardiographic evaluation of patients with tetralogy of Fallot who were surgically corrected. It would also help assess the best timing for further non-invasive imaging procedures, especially MRI.
Limitations
The main limitation of the present study was its retrospective nature; however, the same investigator consulted all clinical files, and all the examinations were performed with a maximum interval of 12 months. This sample may present a subgroup of patients with more advanced disease, in which a complete evaluation by echocardiography, treadmill exercise testing, and cardiac MRI was deemed necessary; however, this is exactly the subgroup where clinical decision for re-operation has to be considered and where the additional value of right ventricular longitudinal strain may be of use. Another limitation is the use of software conceived for the assessment of left ventricular myocardial contractility. In the future, dedicated software should be validated for right ventricular assessment.
Acknowledgements
None.
Authors’ Contributions: All authors have contributed to the study design and data acquisition, analysis, and interpretation. The manuscript was reviewed and approved by all authors.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.








