Introduction
Although the frequency of acute cellular rejection has declined over time in heart recipients,Reference Rossano, Dipchand and Edwards1,Reference Gossett, Canter and Zheng2 antibody-mediated rejection appears to be more prevalent and difficult to manage. In both children and adults, acute antibody-mediated rejection can occur early as well as late after heart transplant affecting 10–20% of patients and accounting for 35% of rejection episodes in contemporary cohorts.Reference Kobashigawa, Crespo-Leiro and Ensminger3,Reference Thrush, Pahl and Naftel4 Antibody-mediated rejection is associated with worse patient and allograft outcomes. The pathologic features of antibody-mediated rejection also do not always correlate well with clinical presentation as haemodynamic compromise can occur with only mild features of antibody-mediated rejection by pathology.Reference Vaughn, Jorgensen and Law5,Reference Everitt, Hammond and Snow6 The overall frequency of rejection with haemodynamic compromise has not decreased significantly in the current era in the paediatric experience;Reference Everitt, Pahl and Schechtman7 one speculation is that some of these episodes are attributable to under-recognised antibody-mediated rejection.
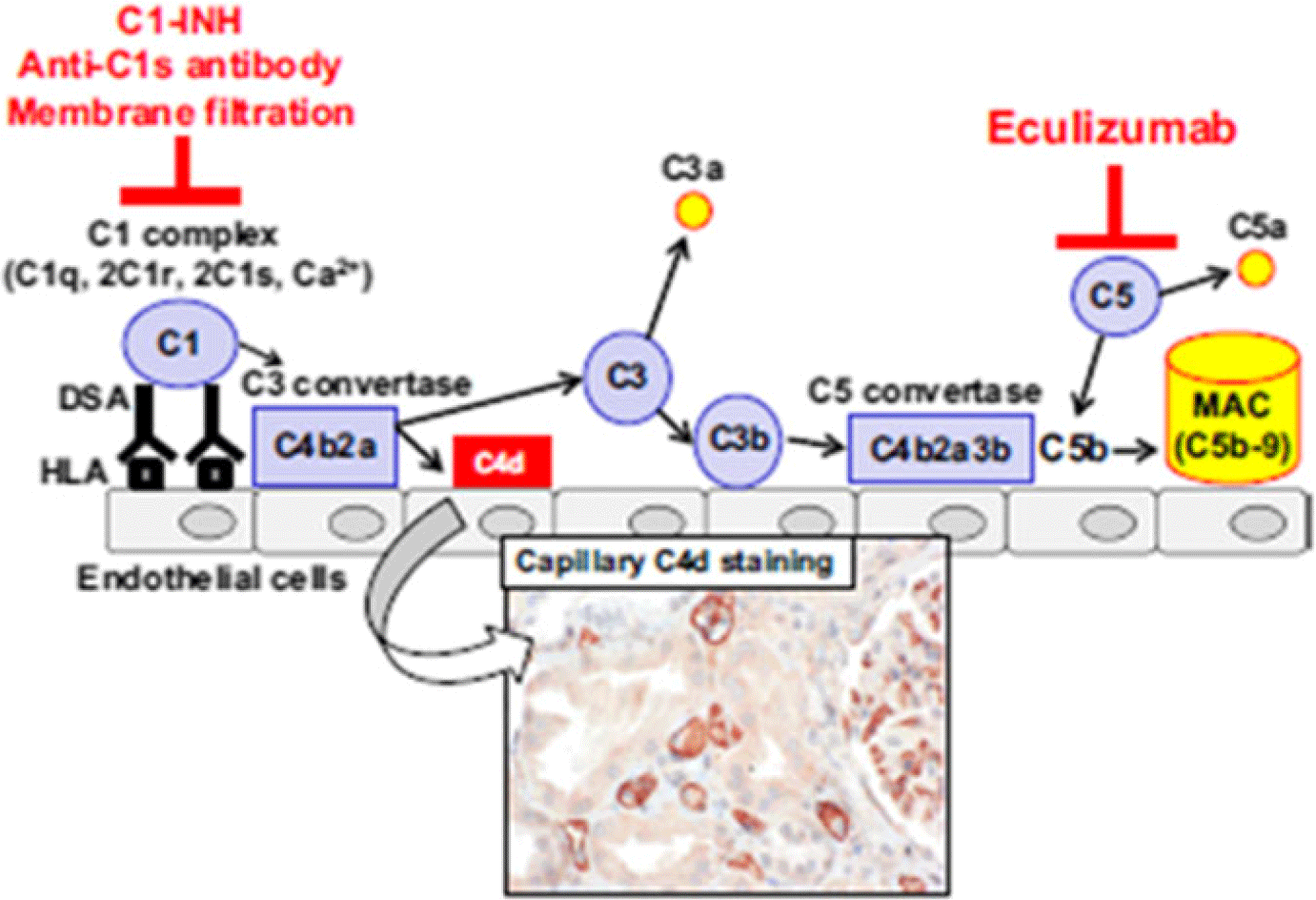
One possible explanation for haemodynamic compromise and worse outcome in antibody-mediated rejection is that drugs targeting the cognate humoral response to human leucocyte antigens, in both the acute and prevention phase of rejection, have not been as effective as those targeting the T cell response responsible for acute cellular rejection. A broad spectrum of therapies is typically used in the treatment of antibody-mediated rejection and includes combinations of intravenous immunoglobulin, steroids, anti-thymocyte globulin, lymphoid irradiation, bortezomib, rituximab, plasmapheresis, and recently eculizumab.Reference Kobashigawa, Crespo-Leiro and Ensminger3,Reference Thrush, Pahl and Naftel4,Reference Colvin, Cook and Chang8 Eculizumab has the advantage of binding to terminal complement C5 preventing its conversion to anaphylatoxin C5a, C5b, and the formation of the cellular damaging membrane attack complex (see Fig 1). Such blockade should reduce endothelial damage as well as decrease recruitment of innate and antigen-specific cell types to the allograft. This blockade can be delivered acutely and maintained over long periods of time with good clinical experience in its use in atypical haemolytic uremic syndrome and paroxysmal nocturnal haemoglobinuria.Reference Olson, Lu and Sulpizio9 Given the difficulty in managing antibody-mediated rejection, the paucity of data with eculizumab in the heart transplant population, its potential benefits but without clinical trials planned with this drug in children, we describe our experience with the use of eculizumab in a multi-centre case series.

Figure 1. Illustration of the components of the complement cascade involved in antibody mediated rejection. Eculizumab inhibits C5 conversion to C5b and membrane attack complex (MAC). From Eskandry F, et al. Transplant International 2016; 29: 392–402.
Patients and methods
This was a descriptive case series of 14 paediatric recipients who received eculizumab for either prophylaxis, as in the case of human leucocyte antigen sensitisation, or treatment of antibody-mediated rejection from 6 paediatric centres between 2010 and 2018. After local institutional review board approval was obtained (where required), detailed case summaries were collected from the medical record. All patients who received eculizumab at these centres for rejection management were reported in this study. The method of direct donor-specific crossmatch testing, panel-reactive antibodies, and donor-specific antibodies differed among the centres, as did the cut-offs for clinical significance, for example, such as leading to stratification in management or intervention. Hence, a positive interpretation is based on each centre’s standards of measurements of anti-human leucocyte antigen antibodies. For the purposes of this paper, the diagnosis of antibody-mediated rejection was standardised to the International Society for Heart and Lung Transplantation working formulation for pathologic antibody-mediated rejectionReference Berry, Burke and Andersen10 as well as clinical evidence for antibody-mediated rejection – if patient received specific anti-antibody-mediated rejection therapy. Haemodynamic compromise was defined as the need for inotropic or mechanical circulatory support. The dosing of eculizumab follows that prescribed for its approved use in the United States of America which is based on weight, induction/maintenance phase, and if plasmapheresis or plasma exchange is performed during the course of eculizumab (Table 1). It is important to note that supplemental doses were given immediately after each plasmapheresis/exchange session as described under Table 2.
Table 1. Dosing regimen of eculizumab directly adopted from its use in atypical haemolytic uremic syndrome (aHUS). From Alexion Pharmaceuticals, Inc. (Boston, MA, USA) http://alexion.com/Documents/Soliris_USPI.aspx

1 Website address provided.
Table 2. Supplemental dosing regimen of eculizumab with plasma exchange. From Alexion Pharmaceuticals, Inc. (Boston, MA, USA) http://alexion.com/Documents/Soliris_USPI.aspx

* PE/PI = Plasmapheresis or plasma exchange; or fresh frozen plasma infusion.
Measure of central tendency was performed with SPSS v. 19 (Armonk, NY: IBM Corp) with median and range for continuous characteristics and absolute with percentage for categorical characteristics.
Results
Clinical characteristics
There were 14 patients (3 females) included in this series. They ranged from 0.8 to 16.3 years of age and were a median of 5.9 years old at the time of transplant. The racial and ethnic make-up was predominantly Caucasian non-Hispanic (n = 10). CHD (79%) was more frequent than cardiomyopathy (21%) as the underlying pre-transplant diagnosis. Eleven (83%) were sensitised to human leucocyte antigens and six (43%) had a positive donor-specific crossmatch at the time of heart transplant. Of the positive crossmatches, three were interpreted to be the result of human leucocyte antigen class I antibodies, two of human leucocyte antigen class II antibodies, and one due to a combination of both class I and class II antibodies (Table 3).
Table 3. Detailed description of individual patients in the study.

Ventricular dysfunction refers to systolic dysfunction unless diastolic dysfunction is specified. Autopsy was not performed unless its results are stated under the Clinical Outcome column.
ACR = acute cellular rejection; CAV = cardiac allograft vasculopathy; DSA = donor-specific antibodies; ECMO = extracorporeal membranous oxygenation; HC = haemodynamically compromised; HLA = human leucocyte antigen; MAS = macrophage activation syndrome; pAMR = antibody-mediated rejection by pathology; PP = plasmapheresis; PRA = panel-reactive antibodies; VT = ventricular tachycardia; XM = crossmatch.
1 Metapneumovirus infection requiring ventilator support and progressive multi-focal leukoencephalopathy by brain imaging noted 3 days after first dose of eculizumab.
2 Fever, haematuria, gastrointestinal bleed.
The indications for eculizumab were acute antibody-mediated rejection with haemodynamic compromise (n = 9), antibody-mediated rejection without haemodynamic compromise (1), persistent antibody-mediated rejection without haemodynamic compromise (1), primary prevention of antibody-mediated rejection with a positive crossmatch (2), primary prevention of antibody-mediated rejection in a human leucocyte antigen-sensitised patient with a negative crossmatch (1) (Table 3). Among the patients with haemodynamic compromise, three were on mechanical circulatory support at the time eculizumab was started. All patients had evidence of antibody-mediated rejection by pathology from endomyocardial biopsy except for the three who received eculizumab for primary prevention. Of the 11 patients with endomyocardial biopsy available before initiation of eculizumab, 5 had pAMR1, 6 had pAMR2, and none had pAMR3. Of the 10 with acuteantibody-mediated rejection, 9 had features of haemodynamic compromise including 1 patient who presented with active heart failure from significant diastolic dysfunction (Patient 10). All patients had circulating donor-specific antibodies deemed clinically relevant at the time of diagnosis of antibody-mediated rejection and initiation of eculizumab (Table 3).
Administration of eculizumab and other treatments of antibody-mediated rejection
Time from diagnosis of antibody-mediated rejection to incorporation of eculizumab into the treatment regimen varied from 0 to 23 days. The median age at first dose was 6.0 years (1–20 years), weight was 17.4 kg (8.1–84.5 kg), and body surface area was 0.73 m2 (0.40–2.11 m2). Initiation of eculizumab was a median of 24 days from transplant (perioperatively-9.1 years) with 10 patients receiving eculizumab within 40 days and 4 patients beyond the first year of transplant (see Table 3). There was a wide range of dosing frequency due to concurrent plasmapheresis and duration of therapy based on a centre’s preference for their individual patients. In general, the doses and schedule followed what is used for atypical haemolytic uremic syndrome (Tables 1 and 2). For those who were treated only during the acute phase or as preventive therapy (n = 9), their number of doses ranged from 2 to 11 over a duration of therapy from 4 to 35 days. For those who stayed on eculizumab longer (5), their duration ranged from 6 to 12 months.
Other therapies for antibody-mediated rejection were employed in addition to eculizumab, including steroid (n = 12), anti-thymocyte globulin (9), bortezomib (10), rituximab (12), intravenous immunoglobulin (13), and plasmapheresis (13). The most common combinations were all of the above without anti-thymocyte globulin (6) or all of the above (5).
Outcomes
Seven patients died from sequelae of antibody-mediated rejection at a median time of 21 days (6–189 days) from initiation of eculizumab. One patient experienced sudden cardiac death (patient 5) after discontinuation of eculizumab. Although systolic function had normalised, bilateral atrioventricular valvar regurgitation and active heart failure symptoms persisted. The autopsy showed no antibody-mediated rejection but significant cardiac allograft vasculopathy. Another experienced worsening systolic function 2 weeks after eculizumab treatment followed by a hyperkalemic cardiac arrest. No autopsy was performed (patient 9), but antibody-mediated rejection was presumed present as there was a resurgence of donor-specific antibodies coinciding with decline in systolic function by echocardiography 2 weeks after transplant with positive crossmatch. The other five patients died from multi-organ failure during the course of eculizumab therapy (0.23–2 months). Of these, antibody-mediated rejection was present at autopsy or presumed present by the clinical scenario and short time from diagnosis of antibody-mediated rejection. Eculizumab was also not always initiated at the time of diagnosis of antibody-mediated rejection and in one case not until 42 days later (patient 8). All patients who expired required inotropic support or MCS.
Of the seven patients who survived, none required re-transplantation at latest follow-up. Their duration of follow-up from initiation of eculizumab is longer, median 9.5 months (2–65 months). All had resolution of antibody-mediated rejection by pathology, normalisation of systolic graft function, and discharged from the hospital. Among these, three had antibody-mediated rejection with haemodynamic compromise. None of the survivors required mechanical circulatory support for the treatment of antibody-mediated rejection.
Eculizumab was also used for primary prevention of antibody-mediated rejection. Among them, one died during treatment when antibody-mediated rejection emerged from a positive crossmatch (patient 12) and two survived (patients 13 and 14) with all three already included in the survival/death descriptive above. Of the two who survived, patient 13 had a positive crossmatch and patient 14 was sensitised but had a negative crossmatch.
While adverse event data were not systematically collected, medical record review demonstrated at least two major infectious events. Patient 1 developed metapneumovirus pneumonitis that resolved, and patient 10 developed invasive fungal infection which was a contributing cause of death. Significant bone marrow suppression occurred in two patients, either from eculizumab or concomitant medical therapy (patients 10 and 14). Patient 1 also experienced multi-focal leukoencephalopathy during metapneumovirus infection. In this patient, the encephalopathy resolved without sequela. The complications of hyperkalemic cardiac arrest (patient 12), oliguria (patient 8), and multi-organ failure eventually leading to death are more likely acute kidney failure after the development of cardiogenic shock; hence, it would be difficult in this setting to attribute them as possible adverse effects of eculizumab. It is important to note that there was no anaphylaxis, blood stream infections, encapsulated bacterial infections, or infusion reactions. The patients who received an extended course of eculizumab, five patients over 6–12 months, did not report any adverse effects related to eculizumab.
Discussion
We describe the use of eculizumab to treat and to prevent antibody-mediated rejection in paediatric heart transplant recipients across six centres. There was variation in the indication and timing for initiation of eculizumab. Furthermore, due to its descriptive nature and small number of patients, we cannot conclude on the efficacy of eculizumab for the treatment of antibody-mediated rejection. We can state that most of these patients who were selected to be treated with eculizumab were critically ill, including having haemodynamic compromise and multi-organ failure. That 7/14 died without a chance for re-transplantation underscores their critical condition. Even among the three patients who received eculizumab for primary prevention, one expired during the treatment of emergent antibody-mediated rejection. One may speculate that the timing of eculizumab therapy is problematic in the majority of the patients in our case series. Eculizumab may work better as a component of therapy in a patient receiving a positive crossmatch heart transplant.Reference Geft and Kobashigawa11 Although it would be intuitive to commence pre-emptive treatment in a patient with a positive crossmatch transplant, there is no published trial data to support such a practice using any regimen by guidelines.Reference Kobashigawa, Colvin and Potena12 There is an ongoing trial incorporating the use of eculizumab in adult recipients who are highly sensitised as preventive therapy for antibody-mediated rejection early post-transplant.Reference Patel, Dilibero and Kittleson13 Whether the outcome of the 14 patients in our study could have been improved with earlier initiation or with selection of less compromised patients for treatment, either to stabilisation or to remission with recovery of graft function or rejection activity to allow re-transplantation cannot be determined. Nevertheless, given the poor prognosis of antibody-mediated rejection, one has to wonder if the patients who survived without re-transplant would have done so if they did not receive eculizumab. Similarly, if eculizumab had been started along with the multitude of other therapies for antibody-mediated rejection, at time of diagnosis, or even at the emergence of donor-specific antibodies, would the outcome in those who expired be modified?
In critically ill patients, direct side effects from a drug can be difficult to ascertain or even discern. For example, bone marrow suppression and infection are seen during intense augmentation of immunosuppression.Reference Fishman and Rubin14 If the infection is from an encapsulated organism, a direct relationship to the drug would be probable, but this was not observed. Renal failure is not described as a side effect related to the drug; however, multiple patients developed cardiogenic shock and acute renal failure. Progressive multi-focal leukoencephalopathy is not a “Boxed Warning” for eculizumab but it is seen in immunocompromised patients and with viral infections and is described in a single case report in a patient with haemolytic uremic syndrome.Reference Gómez-Cibeira, Ivanovic-Barbeito and Gutiérrez-Martínez15 In those patients who were not as compromised such that an independent drug reaction can be identified and who continued to receive many doses over months, no adverse reactions or complications possibly related to the drug were reported. From the safety standpoint, the timing to discontinue eculizumab can be influenced by its good safety track record in haemolytic uremic syndrome, where it is used chronically, and in the limited experience in the current study where five patients stayed on it without complications over 6–12 months. From the efficacy standpoint, our study cannot provide evidence on the timing to discontinue treatment, but one possibility would be to continue until donor-specific antibodies are below clinically significant thresholds, or when features of antibody-mediated rejection have resolved.
The seven patients who survived were less haemodynamically compromised and four did not require inotrope. Two were treated perioperatively with one having a crossmatch. There were five patients in this group that received eculizumab for a protracted period (greater than 6 months). Although we cannot conclude whether their recovery and good outcome was a result of eculizumab without an adequately powered randomised clinical trial, their survival speaks to potential promise in offering these high-risk patients a chance for a good outcome. If eculizumab contributed to a better outcome, it beckons the question of whether eculizumab should be started immediately at time of diagnosis of antibody-mediated rejection as that was not done for all the patients.
In addition, the three patients who were treated to prevent antibody-mediated rejection may be of interest as sensitisation is common in the paediatric population and can increase waitlist time and mortalityReference Feingold, Webber and Bryce16,Reference Sapir-Pichhadze, Tinckam and Laupacis17 as well as being a risk factor for post-transplant mortality.Reference Mahle, Tresler and Edens18 More recently, Webber et al reported a higher incidence of a composite outcome (death, re-transplant, and haemodynamically significant rejection) in recipients with a positive crossmatch heart transplant (18.2%).Reference Webber, Zeevi and Mason19 This observation when compared to the composite outcome of 10.7% in the negative XM group was not statistically significant probably due to the small sample size. These results give hope to the feasibility of transplant across a positive crossmatch for select patients, especially when a better defined antibody-mediated rejection treatment regimen can be formulated as several small studies in the adult renal transplant population have reported promising results in the treatment of antibody-mediated rejection with eculizumab.Reference Stegall, Diwan and Raghavaiah20,Reference Kulkarni, Kirkiles-Smith and Deng21
The cost of eculizumab has been reported at approximately US$46,000 per month of maintenance dosing for an adult-sized patient.Reference van den Brand, Verhave and Adang22 In comparison, four full doses of anti-thymocyte globulin for an adult-sized patient is roughly US$13,000.Reference Zahra, Ayvaci and Michael23 While these costs are extremely high, it is difficult to balance these against the almost unquantifiable price of an allograft and the life expectancy of a recipient. If clinical effectiveness is to be demonstrated, a more thorough cost analysis will be valuable especially if the use of eculizumab is to be extended over many months.
In summary, antibody-mediated rejection remains a major complication after heart transplantation posing a significant clinical challenge, especially in the setting of haemodynamic compromise. Current interventions are not only inadequately studied, but also do not appear to be effective enough. The search for newer modalities to treat antibody-mediated rejection is needed, particularly in preventing antibody-mediated rejection from a positive crossmatch or in improving the outcome of antibody-mediated rejection with haemodynamic compromise.
Author Contribution
Law provided the concept and performed the data collection and analysis; Law, Nandi, and Das drafted the manuscript with further significant revisions provided by Molina; all authors identified appropriate patients and submitted clinical data; all authors reviewed and agreed to the content of the manuscript.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
There are no conflicts of interest to report by the authors.