Transcatheter closure of atrial septal defect (ASD) is a well-established procedure and two major types of ASD devices are currently available in the United States of America. One is the Amplatzer Septal Occluder (Abbott, Abbott Park, IL, United States) and the other is Gore Cardioform ASD Occluder (W.L. Gore & Associates, Inc., Flagstaff, Arizona, United States). These devices have their own delivery and release mechanisms. Particularly, the Gore Cardioform ASD Occluder is known to have a strong tension between the delivered device and delivery catheter. Reference de Hemptinne, Horlick and Osten1,Reference Sommer, Love and Paolillo2 It is not uncommon to observe a significant change of the device orientation upon release of the locking loop. Due to a unique anatomy of ASDs, deficient rims, and device orientation during the delivery, it is sometimes difficult to obtain the proper alignment of the device and atrial septal plane, resulting in device prolapse or malposition. To obtain better device orientation at the atrial septum, different techniques such as using a balloon-assist, steerable catheter, or modifying the delivery sheath to be a side hole sheath have been described. Reference Nounou, Harrison and Kern3–Reference Wahab, Almossawy, Al Bitar and Hijazi6
In this paper, we described the phenomenon that the significant tension from the Gore Cardioform ASD Occluder delivery catheter pulled the properly positioned device from the atrial septum by the retrieval cord upon the release of the locking loop in a child with severe scoliosis. This was overcome by the use of a Mullins sheath to deliver the Gore Cardioform ASD Occluder, resulting in a satisfactory result.
Case report
A 15-year-old male with a history of an orthotopic heart transplant due to restrictive cardiomyopathy was brought to the cardiac catheterisation laboratory for transcatheter closure of his ASD. After his heart transplant, he developed severe antibody-mediated allograft rejection that required extracorporeal membrane oxygenation for severely depressed cardiac function. Due to pulmonary oedema caused by left atrial hypertension, he underwent percutaneous ASD creation by transseptal puncture followed by balloon angioplasty. After recovering from this rejection episode, his ASD remained moderate-sized and was considered for elective transcatheter closure. Complicating his case, he has severe scoliosis.
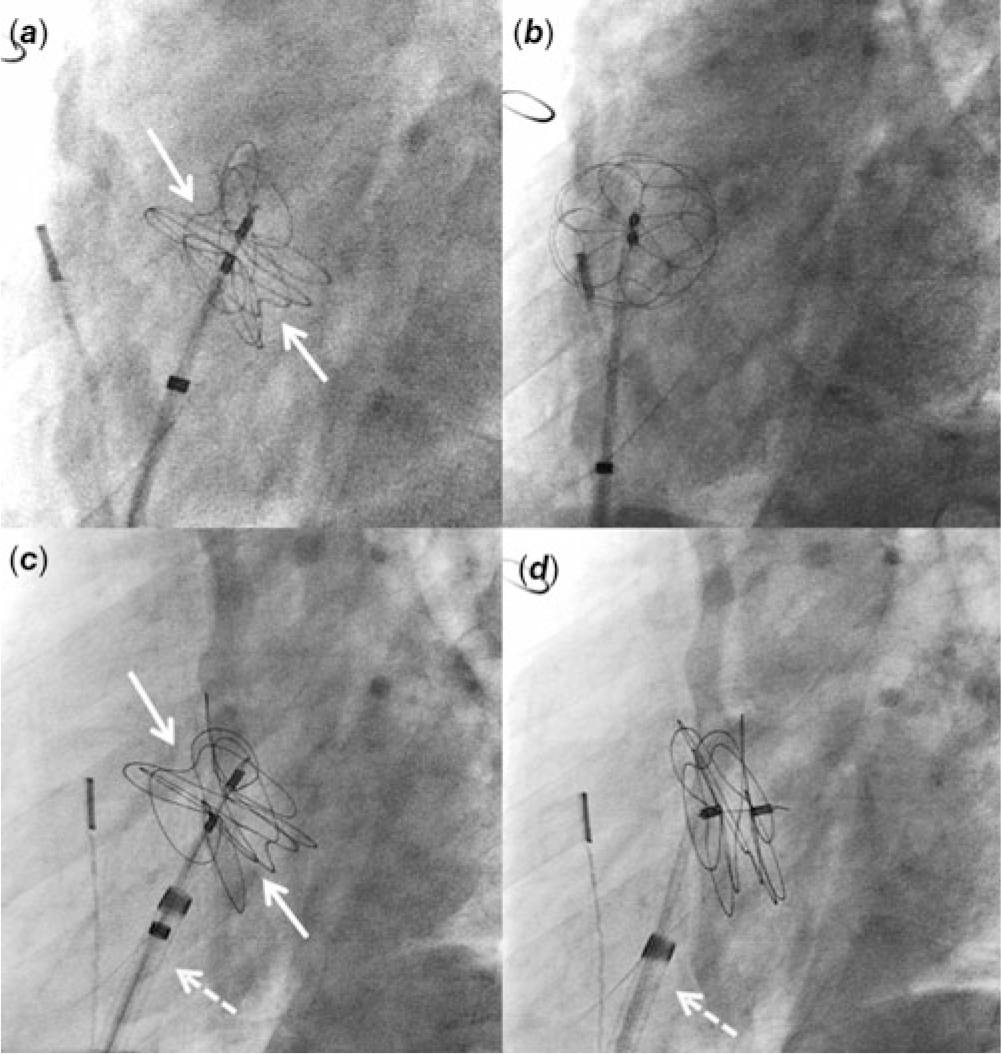
Cardiac catheterisation was performed under moderate sedation with spontaneously breathing. Baseline haemodynamics included a Qp:Qs ratio of 2:1 with a mean pulmonary arterial pressure of 19 mmHg and a pulmonary vascular resistance of 1.2 indexed woods unit. Intracardiac echocardiography (ICE) demonstrated a moderate-sized ASD (static diameter 9.3 mm). This ASD was located at the superior and posterior portion of the atrial septum. Balloon sizing of the ASD showed the waist measuring 16.7 mm. Using an 11 Fr sheath, a 32 mm Gore Cardioform ASD occluder delivery catheter was advanced to the left atrium and the device was delivered under the guidance of ICE. Adequate position was confirmed by fluoroscopy and ICE imaging (Fig 1a). Immediately after the release of the locking loop, the device was pulled into the right atrium by the retrieval cord due to the strong tension of the delivery catheter (Fig 1b; Video 1 in Supplementary material). The device was then removed.
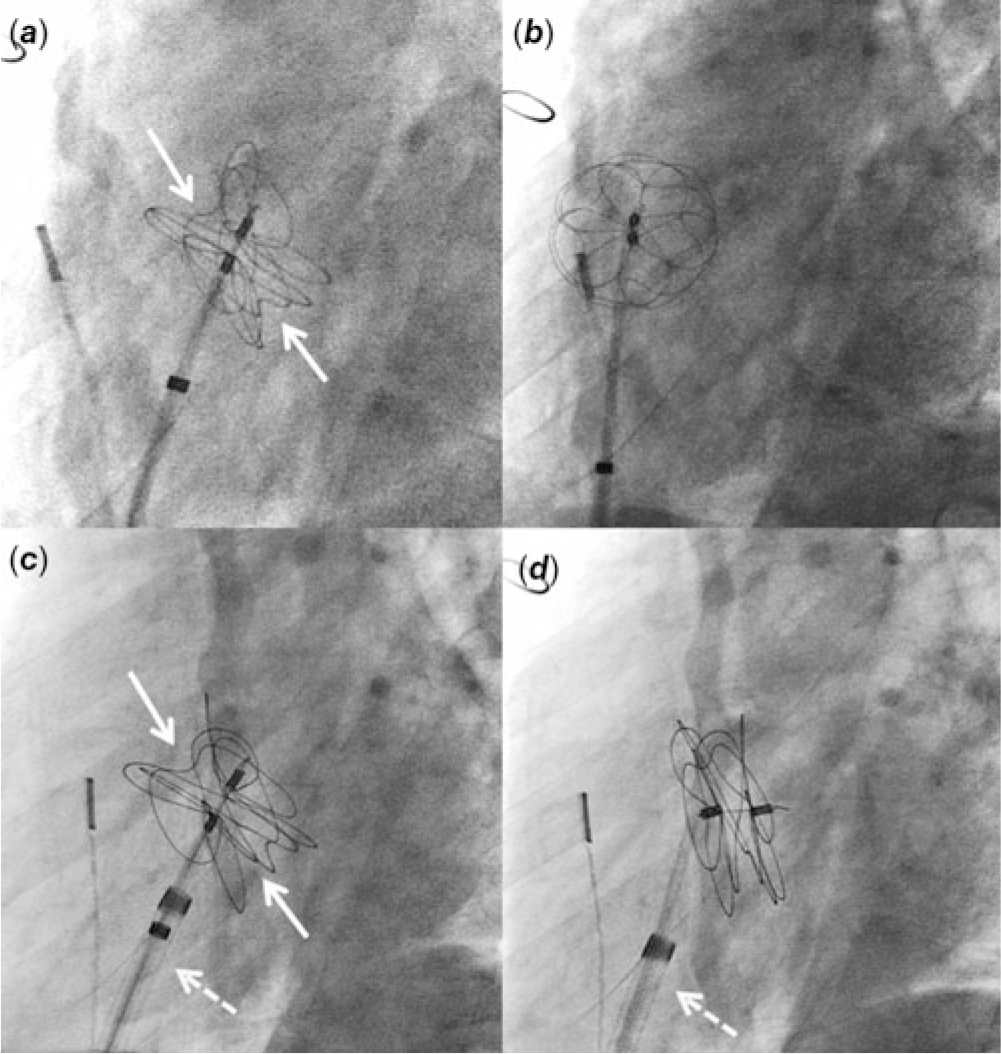
Figure 1. Fluoroscopy imaging at left anterior oblique 60° view. ( a ) A 32 mm Gore Cardioform ASD Occluder is delivered at the atrial septum. There are adequate splays of the device leaflets (arrows). ( b ) After the release of locking loop, the device is pulled into the right atrium by the retrieval cord. ( c ) A 37 mm Gore Cardioform ASD Occluder is delivered at the atrial septum. The delivery catheter is through the 12 Fr Mullins sheath (dotted arrow). ( d ) After the release of locking loop, the delivery catheter remains in the same position due to the support from the Mullins sheath.
To prevent the sudden release of strong tension of the delivery catheter on the device upon locking, a 12 Fr Mullins sheath was used to deliver the device. Although the 32 mm device was adequate for the measured ASD size, the device was upsized to 37 mm to enhance the stability of the device upon its release. Through the Mullins sheath, a 37 mm Gore Cardioform ASD Occluder delivery catheter was delivered at the atrial septum in the same fashion (Fig 1c). The tip of the Mullins sheath was kept towards the atrial septum in the right atrium. The locking loop was released but the device remained at the atrial septum this time (Fig 1d; Video 2 in Supplementary material). The angle of the delivery catheter was held by the Mullins sheath, preventing the acute release of strong tension upon locking. After confirming an adequate position of the device under ICE imaging, the retrieval cord was removed. Final angiography in the right atrium showed a satisfactory position of the device at the atrial septum. At an 8-month follow-up, no device fractures were seen and at an 18-month follow-up, the ASD device was well positioned without a residual shunt.
Discussion
Sudden release of the strong tension between the Gore Cardioform ASD occluder and its delivery catheter caused the device to prolapse into the right atrium. The retrieval cord pulled the device from the atrial septum. This phenomenon occurred because of the unique location of ASD in addition to severe scoliosis in our case. Although the upsized device could be an additional factor of successful device delivery, the use of the Mullins sheath kept the angle of the delivery catheter and prevented the sudden release of tension. This simple technique led to a successful device release in our case.
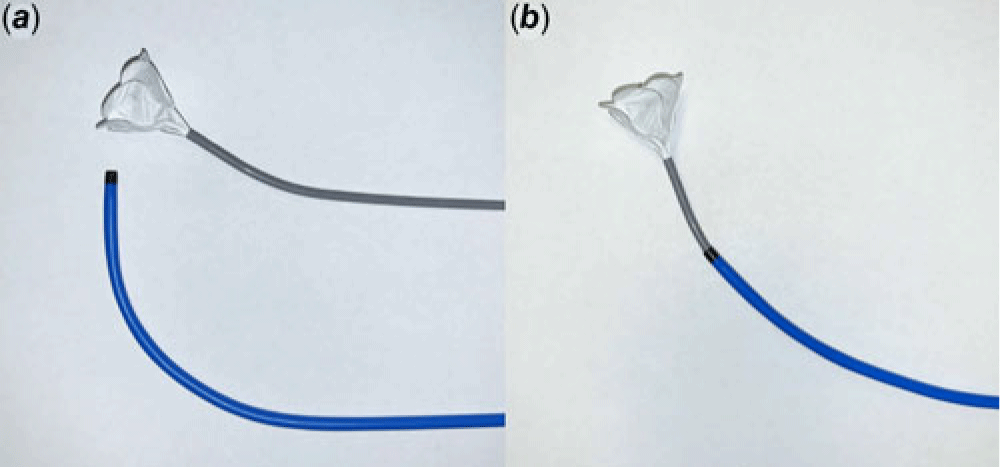
Various techniques to assist in ASD device delivery have been described. Those techniques are mostly used to deliver the device at the atrial septum properly and not to prevent the acute release of tension between the delivery system and the device. Such techniques include the use of a steerable catheter, modifying a Mullins sheath to a side hold sheath, using additional balloon catheters or dilators, directing the delivery sheath to the right upper pulmonary vein, or deploying the left atrial disk very high in the left atrium. Reference Nounou, Harrison and Kern3–Reference Harper, Mottram and McGaw7 In contrast, the Mullins sheath was used to keep the delivery catheter at a suitable angle after the release of the locking loop. This technique is very simple and potentially allows easier delivery of the Gore Cardioform ASD Occluder by achieving a better device septum orientation. The angles of the Gore Cardioform ASD Occluder are compared with and without the use of Mullins sheath (Fig 2). The combination of the Mullins sheath and the delivery catheter provides the device different delivery angles and potentially aids in the device delivery for challenging ASDs with their large size, deficient rims, and unique locations.

Figure 2. Comparison of angles of the Gore Cardioform ASD Occluder and its delivery catheter with and without a Mullins sheath.
In conclusion, the use of a Mullins sheath assisted in the release of the Gore Cardioform ASD Occluder in our case. This technique is very simple and may be considered to deliver the Gore Cardioform ASD occluder in selected cases.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951120004242.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in this case report.