Tracheomalacia from vascular compression is an acquired form of weakness of the trachea that is characterised by an accentuation of the physiological process of “collapse” with expiration. The principal cardiovascular causes in children are a double aortic arch, an abnormal branch of the innominate artery, an anomalous left pulmonary artery, a right aortic arch with ligamentum arteriosus, vascular rings, and left atrial dilatation. The most common associated cardiac abnormalities are patent ductus arteriosus, ventricular and atrial septal defects, abnormalities of the aortic arch, hypoplastic left heart syndrome, hypoplastic right heart, tetralogy of Fallot, dextrocardia, and valvular stenosis. The diagnostic tools used are a helical CT scanner, MRI, and tracheobronchial endoscopy.Reference Austin and Ali 1
Although invasive procedures are not necessary to treat tracheomalacia in the majority of children, respiratory care is essential. There are many options for children who do not recover spontaneously or who have life-threatening symptoms. Tracheotomy and non-invasive ventilation can have important consequences; in fact, they might delay oral feeding and speech, and they might have a detrimental impact on mental development, but are generally considered to be primary treatments or useful adjuvants before other options. Surgery may be necessary in children who experience recurrent pneumonia, intermittent respiratory obstruction, dying spells, or who cannot be extubated.
Tracheal stents are useful because of their less-invasive nature and shorter recovery time, as compared with surgery. Silicon and metal stents are most commonly used, and each has potential complications and limitations. Metal stents have a minimal thickness and are easy to deploy but harder to remove. In addition, they induce the growth of granulation tissue, and additional stent placement or dilatation is often required as children grow. Furthermore, fractures or migrations are rare complications. Silicon stents are easier to deploy and remove if required. The major drawback of these stents is that it is difficult to drain secretions, and a hyperplasic bulge can develop at both ends.
The use of absorbable stents has been studied in animal models, with some relevant reports and case series in humans. Absorbable stents have a similar structure to bare metal stents, allowing direct contact of the mucosa with breathing air. They do not require removal as they are spontaneously absorbed in less than 20 weeks.Reference Austin and Ali 1 – Reference Butler, Speggiorin and Rijnberg 4
Case report
A 28-day-old neonate was admitted to our ICU with cardiogenic shock. Echocardiography revealed an anomalous left coronary artery from the pulmonary artery. The child underwent aortocoronary connection by direct re-implantation.
After extubation, the postoperative period was complicated by an impossibility to wean the child from the non-invasive ventilation, as spontaneous breathing was accompanied by respiratory distress with acidosis. Bronchoscopy showed a moderate-grade tracheomalacia in the distal trachea. The difficulty to wean persisted, and the child was re-intubated following a dying spell; chest CT scan identified a double aortic arch. The surgeon corrected the vascular malformation, but it was still impossible to wean the patient from mechanical ventilation and tracheomalacia persisted. A tracheotomy was therefore performed.
After 2 months in the ICU, the patient’s respiratory condition did not meaningfully improve, and therefore we elected to consider tracheal stenting. We excluded the use of metallic and silicon stents because of the well-known possible complications; therefore, we decided to use an absorbable stent to gain time and allow the child to grow. We used an oversized device in order to cover the entire trachea from the tracheostomy to the carina. We obtained a specifically informed written consent for tracheal stenting from the child’s parents.
The custom-made prosthesis was a self-expanding, biodegradable polydioxanone stent (ELLA-CS, Hradec-Kralove, Czech Republic);Reference Antón-Pacheco, Comas and Luna 5 , Reference Antόn-Pacheco, Luna and Garcıa 6 the stent was 10 mm in diameter and 30 mm in length when expanded. The dimensions of tracheal segments to stent are usually extrapolated by analysing chest CT imagesReference Grillo 7 and broncoscopy. We used the images obtained by bronchography with the patient breathing spontaneously. It was performed in order to visualise the tracheal malacic segment in “real-time” and achieve a measurement as close to reality as possible. The procedure was performed in the angiography room, with the stent released under fluoroscopic and bronchoscopic vision.
The stent was delivered in an airtight container to prevent degradation by humidity. It was loaded manually into a delivery device with a hollow plastic guiding tube with an olive at the end, which accepted a 0.035-inch guidewire, and an over-tube of 5-mm diameter (Fig 1). As we were using rigid bronchoscopy, ventilation was possible during nearly every phase and only a limited period of apnoea (45 seconds) was required.

Figure 1 The free lumen, showing perfect placement of the stent (second bronchoscopy, taken on the day of stent placement).
The procedure was performed under general anaesthesia with propofol, fentanyl, and rocuronium, as well as local lignocaine. After insertion of the prosthesis, the tracheostomy tube was removed and the child received mask ventilation until spontaneous breathing was resumed after propofol interruption and administration of sugammadex. After 30 minutes in the recovery room, the child was transferred to the paediatric ICU awake and with spontaneous ventilation. She did not require respiratory support at any point.
The child underwent bronchoscopy 1 week after stenting, and was discharged after 2 weeks. Bronchoscopy was repeated at 4, 8, and 12 weeks (Fig 2). At week 8, we noted that the stent was almost reabsorbed. We performed tracheal “coring out” to remove any small fragments; no evidence of substantial narrowing of the airways was detected.
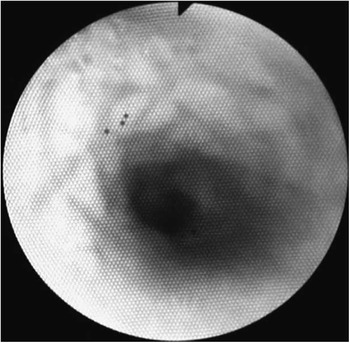
Figure 2 The stent was completely reabsorbed. The tracheal mucosa has a cobblestone aspect. Mild tracheomalacia remains, and rare but unobstructive granulomas can be seen near the carina (sixth bronchoscopy), 12 weeks after device implantation.
The patient had noisy breathing for 6 months, but all of her symptoms gradually disappeared. During the follow-up period, we decided to perform a more invasive investigation only if clinical conditions required it. At 13 months of age, the child was asymptomatic without medical treatment, she weighed 9.4 kg for near 68 cm of height. At 2 years of age, the clinical conditions were really very good.
Conclusions
When deciding to place a stent in a child’s trachea, clinicians must consider whether the procedure is clinically indicated, and, second, the type of stent that should be used.
In this patient, our goal was principally to gain time and allow her to grow and be weaned from mechanical ventilation. We were not sure that the device would be needed long term, and therefore decided to use a completely absorbable stent. This choice was successful. As the prosthesis was oversized, it did not migrate from its optimal positioning. In addition, we did not observe a lot of granulations – in contrast to metal stents – and the child did not need further endoscopic procedures to place another device.
The choice to use an absorbable, oversized tracheal stent allowed a very difficult clinical problem to be managed through only a single procedure, but we are sure that more studies and wider experience will be necessary to define and confirm the best use of absorbable stents in treating tracheobronchial malformations in children. A limited number of previous reports in the literature describe the placement of absorbable stents for various forms of tracheal stenosis.Reference Antón-Pacheco, Comas and Luna 5 , Reference Antόn-Pacheco, Luna and Garcıa 6 , Reference Vondrys, Elliott, McLaren, Noctor and Roebuck 8 The focus of our case report is on the use of tracheal stenting to treat dynamic narrowing caused by tracheal malacia: in this regard, we agree with Antόn-Pacheco et alReference Antόn-Pacheco, Luna and Garcıa 6 that this kind of stent offers a new perspective.
They reported that absorbable stents were not difficult to insert in the trachea and were safe and effective.Reference Antón-Pacheco, Comas and Luna 5 , Reference Antόn-Pacheco, Luna and Garcıa 6 Moreover, we believe they are more versatile and, eventually, useful in several clinical scenarios where metal stents prove to be suboptimal and expose patients to the risk connected to repeated procedures.
Acknowledgements
Cristian Mirabile is the guarantor of the content of this manuscript, including the data.
Authors’ contributions: G.D.D. and C.M. had full access to all data in the case report and take responsibility for the integrity of the reported data. The authors contributed substantially to write the manuscript. This manuscript has not been previously published or printed.
Financial Support
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The infant were treated only after obtaining written parental consent.




