Case details
A full-term male neonate was born to a 25-year-old primigravida. He presented with cyanosis, feeding diaphoresis, and failure to thrive. Pertinent physical examination revealed flat facies, telecanthus, clinodactyly, and muscle hypotonia. Echocardiography (Fig 1) revealed d-transposition of the great arteries (image A), adequate atrial septal defect, patent ductus arteriosus, and a small perimembranous ventricular septal defect. The neonate was planned for arterial switch operation; however, on preoperative evaluation, his complete blood count showed hyperleucocytosis of 122 × 103/µl with normal haemoglobin and platelet count. He also had hyperuricaemia with deranged renal function tests. Blood film (image B. 1–4; ×1000, May Grunwald Giemsa stain) revealed an increased number of blasts (47%) (image 1) and presence of giant platelets (image 2). The blast cells showing characteristic cytoplasmic “blebs/pseudopods” (images 2 and 3) and a micromegakaryocyte (image 4) were also noticed. Flow cytometry studies showed expression of CD34, cytoplasmic CD41, and CD61 antigens on the blasts. Karyotype analysis revealed 47,XY,+21 (image 5), and sequencing results showed GATA1 mutation in the intron 2 region. Combined with the clinical picture, the haematologic findings were consistent with transient abnormal myelopoiesis in a patient with Down syndrome.
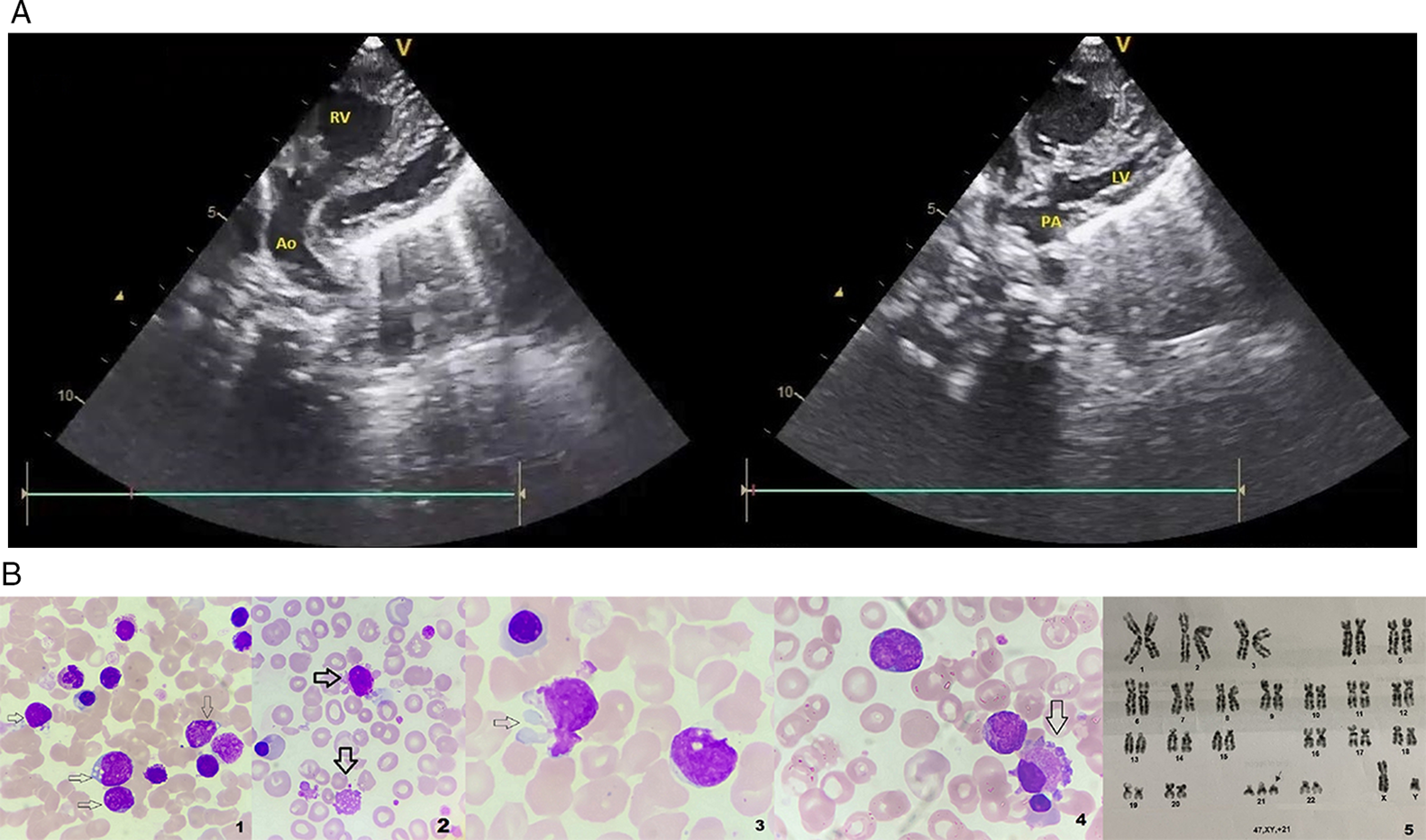
Figure 1. Echocardiography (image A) shows pulmonary artery (PA) arising from left ventricle (LV) and aorta arising from right ventricle (RV). Blood film (image B. 1–4; ×1000, May Grunwald Giemsa stain) shows increased blast cell numbers (multiple arrows, image 1) and the presence of giant platelets (downward arrow, image 2). Blast cells show characteristic cytoplasmic “blebs”/pseudopods (right pointing arrow in image 2 and image 3), and a micromegakaryocyte (arrow, image4) is also seen. Karyotype findings show 47,XY,+21 (arrow, image 5).
Our patient, a term male neonate, was planned for an arterial switch operation; however, surgery was deferred in view of high blast count due to transient abnormal myelopoiesis. He was started on intravenous hydration, hydroxyurea, allopurinol, and low-dose cytarabine to reduce the blast count. Cyanosis kept on worsening despite an adequate atrial septal defect, and the child developed recurrent admissions for chest infection and heart failure, eventually succumbing to his illness.
Discussion
Down syndrome is a chromosomal disorder first described by the French scientist Seguin in 1846. Reference D’Alto, Di Marco, Dimopoulos and Diller1 However, its classical clinical features were first described by an ophthalmologist named Haydon Down in 1866. Reference D’Alto, Di Marco, Dimopoulos and Diller1 On one hand, advanced maternal age is a known factor for an increase in the number of live births with Down syndrome, while on the flip side, improvements in antenatal diagnosis have led to an increase in medical termination of Down syndrome pregnancies. Reference Morris and Alberman2 In most cases, the disorder is caused by chromosome 21 trisomy and less frequently by Robertsonian translocations, isochromosome, or ring chromosomes. Reference Delabar, Theophile and Rahmani3 Down syndrome is associated with a wide spectrum of comorbidities like CHDs, systemic arterial hypertension, pulmonary hypertension, craniofacial and physical abnormalities, gastrointestinal problems, transient abnormal myelopoiesis, leukaemia, cancer, Hirschsprung’s disease, and Alzheimer’s disease. Reference D’Alto, Di Marco, Dimopoulos and Diller1 Transient abnormal myelopoiesis is a unique disorder of Down syndrome and occurs in about 10% of Down syndrome newborns. The majority of newborns are asymptomatic with spontaneous resolution of the disorder after two to three months. Reference Baumann, Niemeyer, Brunning, Swerdlow, Campo, Harris, Jaffe, Pileri, Stein and Thiele4 However, approximately 10% of affected infants die from hepatic or multi-organ failure. About 20% of patients with transient abnormal myelopoiesis that survive beyond neonatal period subsequently develop myeloid leukaemia associated with Down syndrome. The molecular hallmark of transient abnormal myelopoiesis and myeloid leukaemia due to Down syndrome is the presence of somatic mutations in the gene encoding the key megakaryocytic/erythroid transcription factor GATA1 in all cases. Reference Baumann, Niemeyer, Brunning, Swerdlow, Campo, Harris, Jaffe, Pileri, Stein and Thiele4 Blast cells are highly susceptible to low-dose cytarabine chemotherapy. Reference Watanabe5
CHD is commonly seen in patients with Down syndrome. The incidence of CHD in newborns with Down syndrome is up to 50%. Septal defects are commonly seen with atrioventricular septal defect being the most common cardiac abnormality, followed by ventricular septal defect, atrial septal defect, patent ductus arteriosus, tetralogy of Fallot, and multiple abnormalities (coarctation of aorta, pulmonary valve stenosis, or a vascular ring). Reference Versacci, Di Carlo, Digilio and Marino6 Although transposition of the great arteries is one of the most common types of cyanotic CHD, it is uncommonly associated with genetic syndromes, such as Turner syndrome, Noonan syndrome, Williams syndrome, or Marfan syndromes, and in Down syndrome, transposition of the great arteries is virtually absent. Reference Unolt, Putotto and Silvestri7 Genetically, a cell adhesion molecule at chromosome 21q22, CRELD1 (cysteine-rich with epidermal growth factor-like domains 1), and GATA4 genes has been implicated in the development of Down syndrome-related CHD. Reference D’Alto, Di Marco, Dimopoulos and Diller1,Reference McCrossan and McCay8 Furthermore, conotruncal defects like transposition of the great arteries have been found to be associated with chromosomes 10q26 and 13q13. Reference McCrossan and McCay8 Therefore, the probability of acquiring both Down syndrome and transposition of the great arteries together is very small. Only one isolated case of Down syndrome and transposition of great arteries has been previously reported in literature. Reference McCrossan and McCay8 Even though transposition of great arteries could have occurred coincidentally in this infant with trisomy 21, an additional or a combination of different mutations could have resulted in the conotruncal abnormality. Progressive cyanosis, heart failure, and the delayed arterial switch surgery eventually led to the demise of the child. Other factors that may have been relevant include increased pulmonary blood flow due to transposition of the great arteries and the need for immunosuppressive therapy predisposing to infections.
In our case, the child had transient abnormal myelopoiesis and multiple cardiac anomalies including transposition of the great arteries. Both transient abnormal myelopoiesis and CHD are known to contribute to the development of pulmonary hypertension and eventually pulmonary vascular disease (Eisenmenger syndrome) in Down syndrome that adversely affects prognosis and quality of life. Children with Down syndrome and CHD develop progressive pulmonary vascular changes earlier than those without Down syndrome with similar CHD due to intrinsic endothelial dysfunction of the pulmonary vasculature and elevation of plasma levels of asymmetric dimethyl arginine. Reference Cua, Rogers and Chicoine9 Down syndrome may accelerate the process of irreversible pulmonary hypertension in transposition of great arteries.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
Written informed consent was taken from the parent of the patient detailed in the report.




