Introduction
Isolated tricuspid regurgitation other than Ebstein’s anomaly was rare and only a few cases had been reported in children.Reference Schwartz, Gauvreau, del Nido, Mayer and Colan1–Reference Aaron, Mills and Lower6 It was generally believed that isolated tricuspid regurgitation can be well tolerated and they should be treated by drug therapy instead of surgery during infancy and childhood.Reference Morgan and Forker7,Reference Katogi, Aeba, Ito, Goto, Cho, Ueda and Kawada8 However, Aaron BL et al reported that not all children responded well to drug therapy and some children died from chronic cardiac failure or pneumonia.Reference Aaron, Mills and Lower6 Unlike numerous reports on the treatment for isolated tricuspid regurgitation in adults,Reference Lee, Song, Park, Lee, Kang and Song9–Reference Hamandi, Smith and Ryan11 there were only few paediatric cases reported.Reference Patane, Marte, Di Bella, Di Tommaso, Pagano and Chiribiri4,Reference Katogi, Aeba, Ito, Goto, Cho, Ueda and Kawada8,Reference Lim, Huh and Jun12 There was a doubt about whether surgical intervention was required and when surgical intervention was seasonable in children. The purpose of the study was to investigate the clinical characteristics and assess the results of tricuspid valve surgery for isolated tricuspid regurgitation in children.
Methods
Patients
This study was conducted at department of paediatric cardiac surgery, Fuwai Hospital in Beijing, China. A retrospective review of demographics characteristics, operative record, and outpatient clinical data was performed according to approval from our institutional ethics committee. The need for individual patient informed consent was waived. From January 2010 to June 2019, 10 consecutive patients who underwent tricuspid valvuloplasty for isolated tricuspid regurgitation in Fuwai hospital were included. Diagnosis of tricuspid regurgitation was based on clinical evaluation and echocardiography. It was quantified by qualitative and semi-quantitative measurement, which was graded as none (absence of tricuspid regurgitation), mild, moderate, and severe.Reference Lancellotti, Moura and Pierard13 All the patients showed signs of weakness, inappetence, or mild oedema and received drug treatment. As mentioned, indications for tricuspid valvuloplasty were restricted to patients with severe symptoms but unresponsive to drug therapy. Patients who combined with atrial septal defect, ventricular septal defect, or any other cardiac anomalies were excluded.
Endpoints
The primary endpoint was the incidence of moderate or severe tricuspid regurgitation at the latest follow-up. Early death was defined as occurring within 30 days of surgery. Late death was defined as occurring >30 days after surgery. The latest follow-up period was October 2019.
Surgical technique
Tricuspid valvuloplasty was performed through a median sternotomy under cardiopulmonary bypass and standard cannulation of ascending aorta and bicaval cannulation. After the distal ascending aorta was clamped, antegrade cold crystalloid cardioplegia solution was infused into the aortic root. Then, the right atrium was opened and the tricuspid valve was inspected. The annulus, leaflets, and sub-valvar apparatus were carefully evaluated to determine the precise anatomy of the lesion with the aid of saline injection test. Various repair techniques were applied according to pathologic lesions of tricuspid valve. Repair techniques consisted of three levels including sub-valvular, leaflets, and annulus level. Sub-valvular structures were rehabilitated with mal-connected chordae resection, chordal shortening, or artificial chordae tendineae implantation. Leaflets level included leaflet cleft closure, prolapse leaflet plication, or the application of autologous pericardial tablets to increase the flap area. At last, annuloplasty or De Vega technique was always used to reduce the dilated tricuspid annulus. One technique or several techniques may be used in one patient. Transesophageal echocardiography was also used to evaluate the repair results after cardiopulmonary bypass. If transesophageal echocardiography suggested moderate or severe tricuspid regurgitation, reoperation would be indicated.
Statistical analysis
Continuous variables were expressed as mean±standard deviation and categorical variables were expressed as frequency and percentage. All statistical tests were two-tailed. A p value of less than 0.05 was considered statistically significant. All data were managed and analysed using SPSS 23.0 for windows.
Results
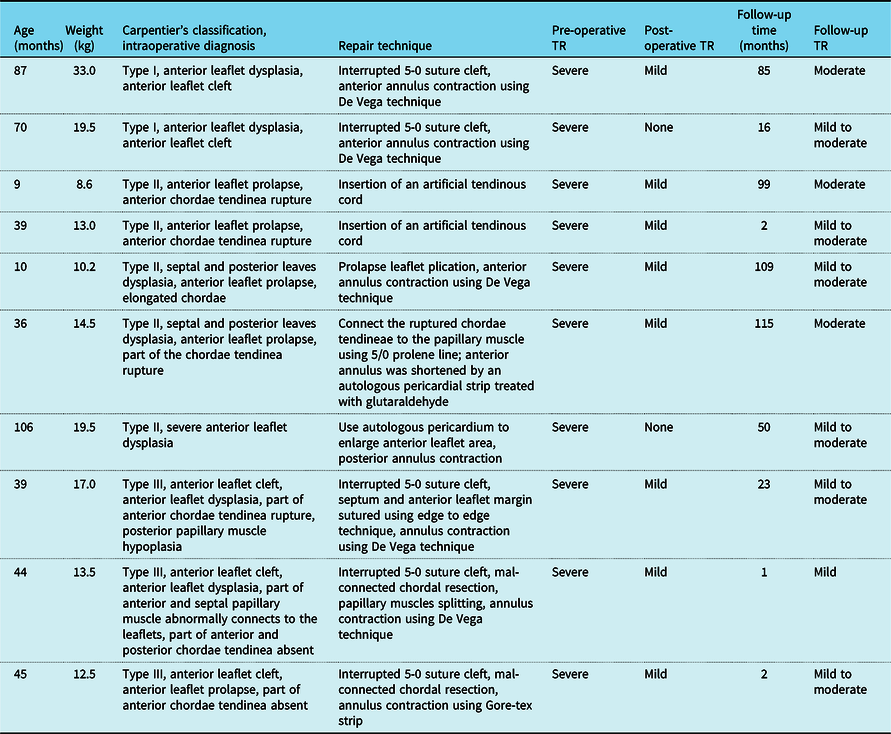
Pre-operative baseline data in our study (n = 10) were summarised in Table 1. All the patients had a history of taking diuretics and digoxin, however, responded poorly to drug treatment and showed severe signs of weakness, inappetence, or mild oedema. There was no better treatment option but surgical intervention. The mean age at repair was 48.5 ± 31.0 (range: 9 to 106) months and the mean weight was 16.1 ± 6.9 (range: 8.6–33.3) kg. Intraoperative tricuspid valve lesions were classified according to Carpentier’s functional classification. There were two (20%) patients diagnosed as type I, five (50%) patients as type II, and three (30%) patients as type III. Almost all the patients combined with leaflets dysplasia, especially anterior leaflet. The main causes for severe isolated tricuspid regurgitation accounted for chordae tendinea rupture (3/10), leaflet cleft (2/10), mal-connected chordal tendinea to leaflets (2/10), severe anterior leaflet dysplasia (1/10), elongated chordae (1/10), and chordae absent (1/10). With different tricuspid valve pathologic morphologies, various repair techniques were used (detailed see in Table 2).
Table 1. Perioperative characteristics

RVEDV = right ventricular end-diastolic diameter, TR = tricuspid regurgitation.
Table 2. Detailed information about pathological classification and repair technique

TR = tricuspid regurgitation.
The mean cardiopulmonary bypass time was 76.7 ± 28.7 minutes and the mean aortic clamp time was 46.3 ± 21.4 minutes. Post-operative echocardiography showed no tricuspid regurgitation in two patients, mild regurgitation in eight patients, and there was no patient with moderate or severe tricuspid regurgitation. All patients detached from respirator successfully, and the mean mechanical ventilation time was 5.9 ± 1.9 hours. The volume of right ventricle decreased after surgery. The mean end diastolic diameter of right ventricle decreased from 19.6 ± 4.8 mm to 15.9 ± 3.9 mm. The cardiothoracic ratios on their chest roentgenograms decreased from 0.59 ± 0.05 to 0.54 ± 0.05. All the patients recovered smoothly and there was no early death.
The mean follow-up time was 50.4 ± 47.2 (range: 1–115) months. The latest echocardiographic data showed mild to moderate tricuspid regurgitation in seven patients, moderate tricuspid regurgitation in three patients, and no patient with severe tricuspid regurgitation. The patients with moderate tricuspid regurgitation were asymptomatic without any discomfort. No patient developed tricuspid stenosis and there was no late death. All the patients were in NYHA functional class I.
Discussion
Isolated tricuspid regurgitation had no displacement of the tricuspid valve and thus no atrialised ventricle, which was quite different from Ebstein’s diseases.Reference Aaron, Mills and Lower6 Children were so rare that there were only few cases reported.Reference Jaquiss and Imamura3–Reference Seguela, Roubertie, Tafer, Thambo and Iriart5,Reference Katogi, Aeba, Ito, Goto, Cho, Ueda and Kawada8,Reference Lim, Huh and Jun12,Reference Mizuno, Hoashi and Sakaguchi14 About 43,000 children diagnosed with CHD underwent cardiac surgery in Fuwai hospital from January, 2010 to December, 2019. However, only 10 consecutive patients with isolated tricuspid regurgitation underwent tricuspid valve repair surgery. Thus, the prevalence was estimated at 10 in 43,000 (0.023%) of CHD population. It was generally acknowledged that isolated tricuspid regurgitation can be well tolerated, and even one patient without clinical symptoms for 32 years.Reference Morgan and Forker7,Reference Beckhoff, Alushi and Jung15 But not all children responded well to drug therapy and some patients died from chronic cardiac failure or pneumonia in previous reports.Reference Aaron, Mills and Lower6 Thus, some patients require timely surgical intervention, repair, or replacement. Tricuspid valve replacement in children was a procedure with a high risk of early post-operative death. Heather et alReference Bartlett, Atkins and Burns16 reported 97 cases of tricuspid valve replacement in children <6 years of age from 1984 to 2002, that 26 patients (27%) did not survive to discharge (n = 24) or required cardiac transplantation before discharge (n = 2, considered failures for analysis) after tricuspid valve replacement. Although 35 patients surviving to discharge with further data available, 13 (37%) patients were known to have died or undergone cardiac transplantation. Hence, we believed that tricuspid valvuloplasty was the best choice for children with the advantages of avoiding anticoagulation and good growth potential. As described in the article, tricuspid valvuloplasty was performed successfully in all of these patients and there was no patient underwent tricuspid valve replacement. As mitral repair, the principles of tricuspid valve reconstruction were restoration of full leaflet mobility, correction of prolapse, provision of a large leaflet coaptation surface, and annular stabilisation.Reference Antunes, Rodriguez-Palomares and Prendergast17 This study demonstrated our experiences of tricuspid valvuloplasty for severe isolated tricuspid regurgitation in children.
There were various pathological lesions may lead to severe tricuspid regurgitation. As explained in our study, major causes for isolated tricuspid regurgitation in paediatrics included congenital leaflet dysplasia and abnormal sub-valvular structure, especially leaflet cleft and abnormal chordae tendineae. Most of the patients were clearly diagnosed in the neonatal period for cyanosis and need surgical repair.Reference Katogi, Aeba, Ito, Goto, Cho, Ueda and Kawada8,Reference Lim, Huh and Jun12,Reference Sachdeva, Fiser, Morrow, Cava, Ghanayem and Jaquiss18 Besides, Morgan et alReference Morgan and Forker7 confirmed that trauma can also lead to papillary muscle or chordae tendineae rupture, leading to severe tricuspid regurgitation.
Thirty per cent of patients in our cohort combined with chordae tendinea rupture, and it was believed to be congenital developmental abnormality on the basis of clinical history. To repair the ruptured chordae tendinea, insertion of an artificial tendinous cord or using 5/0 prolene suture to connect the ruptured chordae tendineae with papillary muscle was performed.Reference Katogi, Aeba, Ito, Goto, Cho, Ueda and Kawada8,Reference Lim, Huh and Jun12,Reference Sachdeva, Fiser, Morrow, Cava, Ghanayem and Jaquiss18 Toshiyuki et al reported their experience of artificial chordae implantation in a 15-year-old boy and recovered well after the operation.Reference Katogi, Aeba, Ito, Goto, Cho, Ueda and Kawada8 Rody et al agreed that tricuspid valve repair with artificial chordae in children demonstrated acceptable results and valvular restriction by the artificial chordae was not observed after mid-term follow-up.Reference Boon, Hazekamp and Hoohenkerk19 Jolanda et al reported their 15-year experience with the use of artificial chords for valve reconstruction in children, and they found that only 1 (6.7%) patient occurred leaflet motion restricted caused by artificial chords. Despite patients grew and developed over time, we agreed that restricted leaflet motion by the artificial chords did not seem to form a major problem.Reference Kluin, Sojak, Koolbergen, Boon, Bokenkamp and Hazekamp20
Besides chordae tendinea rupture (3/10), there were 40% patients in our series diagnosed with abnormal chordae tendinea, including mal-connected chordal tendinea to leaflets (2/10), elongated chordae (1/10) and chordae absent (1/10), and severe anterior leaflet dysplasia (1/10).Tethering of the tricuspid valve leaflets by aberrant tendinous chords can be the sole mechanism of severe congenital tricuspid regurgitation.Reference Kobza, Kurz and Oechslin21 Aberrant tendinous chords connected to the tricuspid valve leaflets would limit the mobility of the tricuspid leaflet and resulted in incomplete coaptation and apical displacement of the regurgitant jet origin.Reference Kobza, Kurz and Oechslin21 Tricuspid structure should be carefully examined by echocardiography before surgery and distinguish it from other malformations involving tethering. In our experience, resection of the tethering chords could restore full mobility to the involved leaflet, which could reach the coaptation plane.
Leaflet clefts in tricuspid valve was a rare disorder with an incidence of 0.6% among patients with CHD.Reference Eichhorn, Suetsch, von Segesser, Turina and Jenni22 In another study, the prevalence was estimated in 5 of 28,091 (0.018%) Doppler echocardiograms. Many previous cases reported it was associated with patent foramen oval, atrial septal defect, peri-membranous ventricular septal defect, or pulmonary stenosis.Reference Seguela, Roubertie, Tafer, Thambo and Iriart5,Reference Eichhorn, Suetsch, von Segesser, Turina and Jenni22,Reference Motoyoshi, Tofukuji, Sakurai, Ohmi and Tabayashi23 The pathogenesis was still unknown. It may be associated with the development of the endocardial cushion.Reference Seguela, Roubertie, Tafer, Thambo and Iriart5 The diagnosis of tricuspid regurgitation can be established by echocardiography and then depicted the tricuspid valve morphology accurately.Reference Seguela, Roubertie, Tafer, Thambo and Iriart5,Reference Motoyoshi, Tofukuji, Sakurai, Ohmi and Tabayashi23 It was effective to control tricuspid regurgitation with only cleft closure and tricuspid annuloplasty in our patients, while Kosuke et al thought it was necessary to perform chordal reconstruction to stabilise the repair and prevent the recurrence of tricuspid regurgitation if the patient diagnosed with remarkably enlarged the anterior leaflet and elongated chordae tendineae.Reference Saku, Inoue, Yamamoto and Ueno24 For patients with severe tricuspid leaflet dysplasia or with inadequate leaflet area, the use of an autologous pericardial patch to enlarge the tricuspid leaflets could increase the surface of coaptation and allow good coaptation effectively at the level of the tethered anterior and posterior leaflets while maintaining leaflet mobility.Reference Dreyfus, Raja and John Chan25
Surgical options were related to specific structural anomalies of leaflets, annulus, and sub-valvar structures. Recognition of tricuspid valve anatomy and precise definition of tricuspid lesion were essential to tailor a specific and appropriate repair surgery. In our experience, if patients with simple or single pathological lesion, such as leaflet cleft, mal-connected chordal tendinea to leaflets, or elongated chordae, they may get a good outcome after surgery. Complex or multiple tricuspid lesions, such as severe anterior leaflet dysplasia, chordae tendinea rupture, or chordae absent, on the contrary, may responsible for increased surgical difficulty and recurrent risk. Besides, the use of autologous pericardial patch to enlarge the tricuspid leaflets and insertion of an artificial tendinous cord may also add the risk of recurrence of valve regurgitation. Since our series were small, larger sample size and longer follow-up were needed.
Conclusions
Isolated tricuspid regurgitation other than Ebstein’s anomaly was associated with structural anomalies of leaflets, annulus, and sub-valvar structure. For patients who were not well responsive to drug therapy, individualised tricuspid valve repair can achieve an excellent result.
Acknowledgements
None.
Financial support
This work was supported by National Key R&D Program of China [2017YFC1308100] and National Natural Science Foundation of China [81570289].
Conflicts of interest
None.
Compliance with ethical standards
This article did not contain any studies with human participants or animals performed by any of the authors.