Published online by Cambridge University Press: 24 May 2005
We report two instances of transient isolated right-sided myocardial hypertrophy in patients with an intact ventricular septum, normal thickness of the posterior wall of the left ventricle, and normal ventricular function, diagnosed by echocardiography on the third day of life. The two neonates, born at 36 and 38 weeks gestation respectively, had perinatal distress. Both were diagnosed as having isolated right ventricular hypertrophy with mild pulmonary hypertension, which disappeared in both cases within 8 weeks without any specific therapy. Though the cause of the ventricular hypertrophy remains unclear, we believe that it is the consequence of remodeling of pulmonary vasculature secondary to acute perinatal distress, resulting in persistent pulmonary hypertension and producing pressure overload on the right ventricle, and hence right ventricular hypertrophy. The finding of early and transient right ventricular hypertrophy, with normal left-sided structures and normal ventricular function, has thus far failed to gain attention in the paediatric cardiologic literature.
The heart is one of the target organs affected by perinatal distress,1, 2 now reported as a cause of transient hypertrophic cardiomyopathy of the neonate.3, 4 Thus, Valliant et al.3 reported three neonates with fetal distress who developed transient hypertrophic cardiomyopathy. They postulated myocardial ischemia as the cause of the hypertrophy. Chen4 subsequently reported a similar case. We describe here two cases of transient isolated right ventricular hypertrophy with normal ventricular function occurring in neonates with perinatal distress born to mothers after uneventful antenatal periods and without any history of diabetes.
We describe two male neonates, appropriate for gestation age, one born at 36 weeks gestation by caesarian section as the third pregnancy, the mother having undergone a previous caesarian section, and the second born as the second pregnancy at 38 weeks gestation by vaginal delivery. There was no history of diabetes mellitus or hypertrophic cardiomyopathy in the mothers of either baby. Both neonates had low Apgar scores, and required bag and mask ventilation for 20 to 30 sec. Subsequently, both babies also developed respiratory distress and cyanosis soon after birth, with no evidence of respiratory or infectious pathology contributing to the symptoms. Arterial blood gases in both cases revealed systemic hypoxia, with saturations of 78% and 84% respectively, and mild retention of carbon dioxide. Other than supportive management with intravenous fluid and maintenance of temperature, the first baby was intubated and ventilated for persistent severe respiratory distress with cyanosis, while the second baby received oxygen by a hood. Neither baby had received steroids.
A cardiological opinion was sought on third day of life for persistent respiratory distress and hypoxia. Echocardiography in both cases revealed marked hypertrophy of the right ventricular free wall and the ventricular septum, with a small right ventricular cavity (Fig. 1). The posterior wall of the left ventricle, and the cavity, were of normal dimensions. There was mild tricuspid insufficiency, with pressure gradients of 32 and 36 mmHg respectively, with right-to-left shunting across a patent oval foramen. No associated structural cardiac abnormality was detected. We made the diagnosis of hypertrophic cardiomyopathy involving predominantly the right ventricle, with mild pulmonary arterial hypertension, and managed the patients conservatively.

Figure 1. A subcostal four-chamber view on the third day of life reveals severe isolated right ventricular hypertrophy. RV: right ventricle; LV: left ventricle; RA: right atrium; LA: left atrium.
Their condition improved gradually, and they were discharged on 10th and 8th days of life, respectively, in stable condition and without any medication. At follow-up at 8 weeks, the babies were stable, with saturations of oxygen in room air of 95% to 98%. Echocardiography now revealed complete regression of the hypertrophy, and normalization of the right ventricular cavity (Fig. 2). By now, the pulmonary arterial pressures had returned to normal, with only a small left-to-right shunt across the oval foramen.
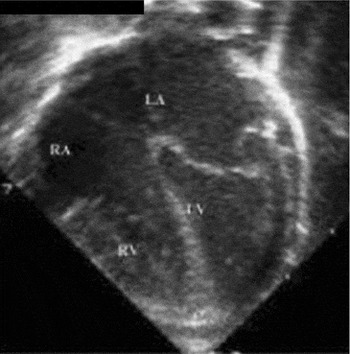
Figure 2. The comparable subcostal four-chamber view at eight weeks of life shows complete regression of the right ventricular hypertrophy. RV: right ventricle; LV: left ventricle; RA: right atrium; LA: left atrium.
Both of our patients presented with cyanosis and respiratory distress in the early neonatal period. Both had a history of perinatal distress, with low Apgar scores, and both needed resuscitation at birth. One of the babies, born at 36 weeks gestation, was given ventilatory support due to persistent respiratory distress and hypoxia, but did not show any improvement with ventilation. The second baby needed oxygen delivered through a hood, the fraction of inspiratory oxygen being 80%. There was no history of diabetes mellitus or hypertrophy cardiomyopathy in either mother, nor evidence of any respiratory pathology or sepsis in either case. Neither baby had received steroid as part of their treatment.
Echocardiographic evaluation revealed hypertrophic cardiomyopathy involving only the right ventricular free wall and the ventricular septum, reducing the size of the right ventricular cavity. There was also mild tricuspid insufficiency and mild pulmonary hypertension. In both the cases, the hypertrophy had regressed by 8 weeks of age, at which stage ventricular function was normal, with only a small left-to-right shunt across the oval foramen.
As described in the literature, the neonatal manifestations of myocardial ischemia have been myocardial necrosis manifesting as cardiac failure and tricuspid and mitral insufficiency.1, 2, 5 As far as we know, transient hypertrophic cardiomyopathy after acute fetal distress was first reported by Vaillant et al.,3 and later by Chen.4 All their babies, however, had left ventricular dysfunction, which finally disappeared in all cases over a period of 1 to 5 months. Manetti et al.6 also reported transient asymmetrical septal hypertrophy in 4 infants less than 9 months of age after hypoxic injury. They postulated an increase in right ventricular systolic pressure, or pulmonary hypertension as the primary stimuluses causing right ventricular pressure load and right ventricular hypertrophy. Experimental studies in rats7–9 have shown that isolated right ventricular hypertrophy can occur after left ventricular mural infarction.
The mechanism producing the transient and isolated right ventricular hypertrophy in our cases is not clear. It may be a mechanical adaptation to pressure overload. Acute fetal distress results in remodeling of pulmonary vasculature, which is responsible for persistent pulmonary hypertension of the newborn, producing hypertrophy of the right ventricle due to pressure overload.5 In both of our patients, however, the degree of hypertrophy was out of proportion to the predicted pulmonary arterial pressures at the time of evaluation, suggesting that other mechanisms might have played a role. Our observations, nonetheless, show that acute perinatal distress can induce transient hypertrophic cardiomyopathy involving predominantly the right ventricle, and with a good prognosis, similar to the hypertrophy cardiomyopathy seen in neonates of diabetic mother.10 This is in contrast to other causes of neonatal hypertrophy cardiomyopathy, which are associated with a poor prognosis. The initial presentation of such babies may mimic cyanotic congenital cardiac disease, a point to bear in mind when seen by the pediatric cardiologist. Careful echocardiographic evaluation, of course, will rule out structural congenital cardiac disease. Serial echocardiograms will then be necessary to document the return of normal physiology and the regression of right ventricular hypertrophy. Our cases suggest that a good prognosis can be predicted for right ventricular hypertrophy subsequent to perinatal distress, even if the hypertrophy is considerable.

A subcostal four-chamber view on the third day of life reveals severe isolated right ventricular hypertrophy. RV: right ventricle; LV: left ventricle; RA: right atrium; LA: left atrium.

The comparable subcostal four-chamber view at eight weeks of life shows complete regression of the right ventricular hypertrophy. RV: right ventricle; LV: left ventricle; RA: right atrium; LA: left atrium.