Aortopulmonary window is a rare cardiac defect representing 0.2–0.6% of all cardiac malformations.Reference Tiraboschi, Salomone and Crupi1 It is a communication between the ascending aorta and the pulmonary trunk and/or right pulmonary branch in the presence of both separate sigmoid valve planes. Early management with surgical or transcatheter routes is lifesaving.Reference Atiq, Rashid, Kazmi and Qureshi2 Here, a case of 9-month-old patient, who underwent a successful percutaneous aortopulmonary window closure with Amplatzer duct occluder II-additional size device, is presented.
Case report
A 9-month-old female patient with growth retardation (height–weight < 3rd percentile) was referred to paediatric cardiology because of cardiac murmur, tachycardia, and tachypnea. She had mild tachycardia (130 beats/minute), tachypnea (40/minute), and a 3rd degree continuous murmur most prominent in pulmonary area. Chest radiography revealed pulmonary congestion and enlarged cardiac silhouette (cardiothoracic index: 0.57). Electrocardiogram showed normal sinus rhythm with a heart rate of 130 beats/minute. Echocardiography revealed dilated left cardiac chambers, mild mitral regurgitation, and a turbulent left-to-right shunt with a diameter of 4.5 mm and a peak Doppler gradient of 60 mmHg between ascending aorta and main pulmonary artery. Left ventricular internal dimension in diastole and left atrial to aortic root ratio was 35.5 mm (M-Mode Z-Score: +6.06) and 1.81, respectively. Cardiac catheterisation was performed under general anesthesia with diagnosis of aortopulmonary window type I for device closure of the defect. The right femoral artery and vein were used; 75 mg/kg unfractionated heparin and 50 mg/kg cefazolin were administered at the onset of the procedure. Aortic pressure was 79/34 mmHg and pulmonary artery pressure was 34/16 mmHg; Qp/Qs and Rp/Rs ratios were >2.5 and 0.21, respectively. Ascending aorta angiogram showed filling of the main and branch pulmonary arteries through a defect of 3.7 mm located 3.5 mm away from the left coronary ostium (Fig 1a and b). The defect was passed from aorta to pulmonary artery using a 4F cobra catheter and guidewire was placed to the pulmonary artery. Over this wire, 4F Amplatzer delivery system was advanced through the aortopulmonary window into the pulmonary artery. The distal disc of a 5 × 2 mm Amplatzer duct occluder II-additional size device was opened in the pulmonary artery. The device was then retracted towards the defect, and the proximal disc was opened on the aortic side of the defect. Because the defect was close to the left coronary ostium, we decided to use a shorter-edged device. The device was released gently after confirming the position by transthoracic echocardiography and aortography (Fig 2). There was not any complication during and after the procedure. Echocardiography showed a well-positioned device with no great vessel obstruction or residual shunt, and the patient was discharged on the following day. At the 2 months’ follow-up visit, the patient had a weight gain of 1200 grams. Echocardiography revealed normal sized cardiac chambers, and there was no mitral regurgitation and great vessel obstruction or residual shunt.

Figure 1. (a and b) Ascending aorta angiogram showed filling of the main and branch pulmonary arteries through a defect of 3.7 mm located 3.5 mm away from the left coronary ostium.
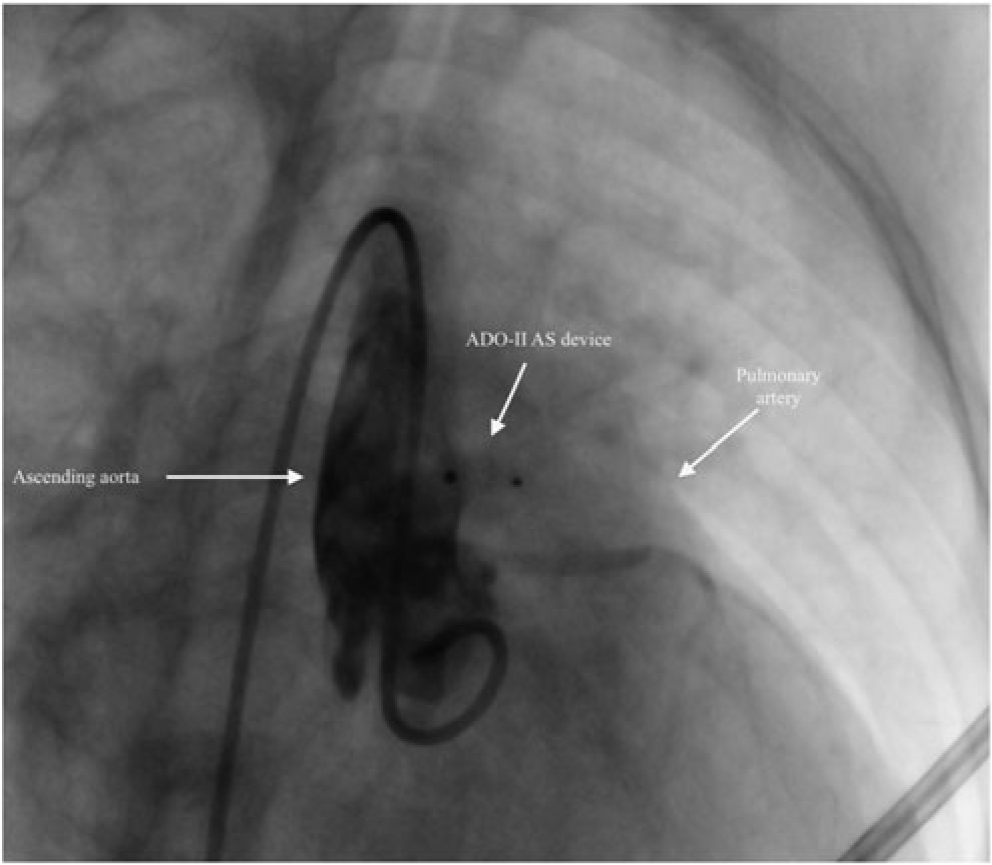
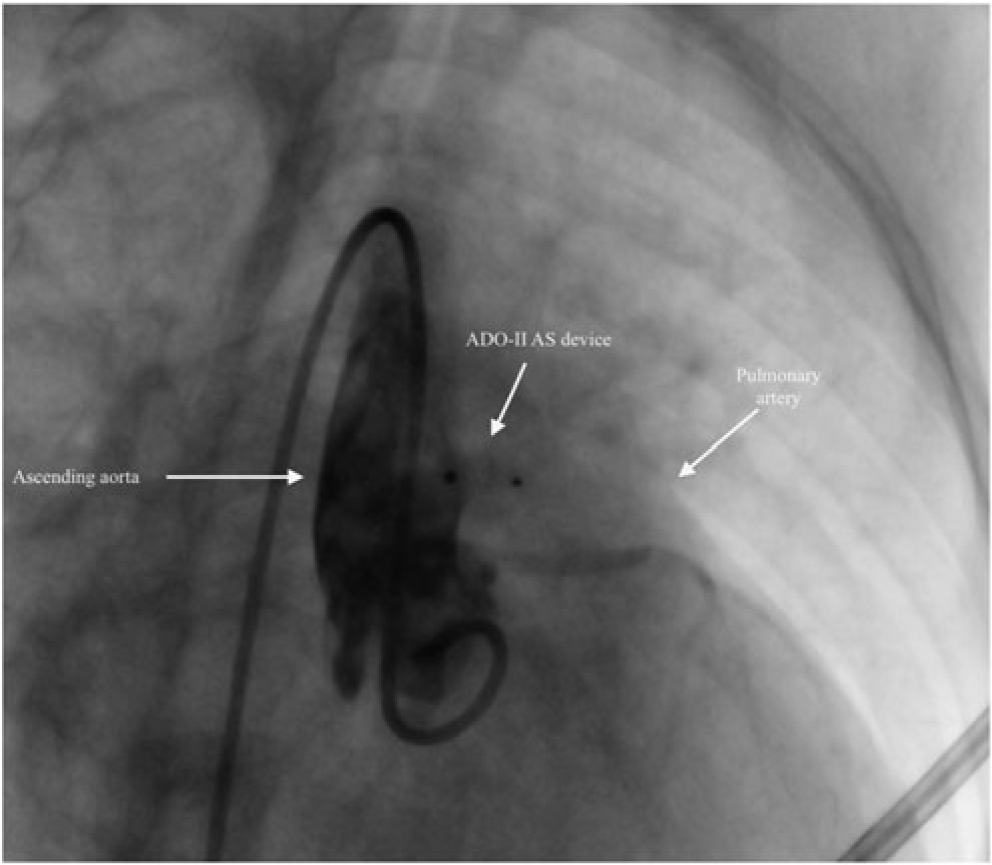
Figure 2. Aortography showing the appropriate position of the device and no residual shunt after transcatheter closure of aortopulmonary window with 5×2 mm ADO II – AS device.
Discussion
Aortopulmonary window represents approximately 0.1% of all CHD. It is a congenital deficiency in septation of the truncus arteriosus, resulting in persistent direct communication between the ascending aorta and the pulmonary trunk.Reference Atiq, Rashid, Kazmi and Qureshi2 Patients with small defects may be asymptomatic; however, symptoms of congestive heart failure and pulmonary hypertension usually develop in patients with large defects.
Traditional treatment of choice is surgical closure of the defect; however, transcatheter closure is minimally invasive, and the risks of cardiopulmonary bypass and bleeding are avoided. Although transcatheter closure may have more advantages, there is limited experience, and it has not become a standard treatment yet. When the defect is relatively small and when the localization of the defect provides sufficient space for the edge of the device, transcatheter closure may be the choice of treatment. Transcatheter closure may be considered only if there is a sufficient distance between the defect, the valves, and coronary arteries, and if there is no other cardiovascular anomaly. The device must be chosen appropriately in accordance with the shape and margin of the defect.Reference Li, Zhu and Feng3 With the advances in transcatheter closure of cardiac defects, transcatheter closure of aortopulmonary window is also being reported recently. Stamata et al. were the first to describe transcatheter closure in a 3-year-old patient using a modified double umbrella occluder system.Reference Stamato, Benson, Smallhorn and Freedom4 Transcatheter closure of aortopulmonary window with a Rashkind double umbrella systemReference Tulloh and Rigby5 and transcatheter closure of residual aortopulmonary window with Amplatzer septal occluderReference Richens and Wilson6–Reference Alkhouli, Tarabishy, Kawsara, Alqahtani and Moiduddin8 and muscular ventricular septal occluder deviceReference Li, Zhu and Feng3 were also reported. Odemis et al. reported a 3-month-old patient, who had a defect of 5 mm, treated successfully with a Nit-Occlud PDA-R device.Reference Odemis, Guvenc, Saygi and Demir9 Usage of Amplatzer-type devices is now well established in the treatment of atrial septal defects, total cavopulmonary connection fenestrations, and arterial ducts.Reference Richens and Wilson6 Although transcatheter closure of aortopulmonary window with various devices has been reported previously, to the best of our knowledge, this is the first case of aortopulmonary window closed with Amplatzer duct occluder II-additional size device. The size and the proximity of the defect to the aortic valves and coronary ostium must be carefully evaluated if transcatheter closure is considered as the choice of treatment. For defects in close proximity to aortic valve and coronary ostium, risk of aortic regurgitation and embarrassment of coronary artery flow will be higher. In patients with a defect in close proximity to coronary ostium or in the infant age group, Amplatzer duct occluder II-additional size device is valuable because of its small disc size.
Conclusion
Transcatheter closure of aortopulmonary defect may be the choice of treatment in patients whose defects are suitable for transcatheter device closure. Various devices, depending on the defect size and position may be used. Amplatzer duct occluder II-additional size device may be considered as the device of choice for defects in close proximity to aortic valve and/or coronary ostium, because it has smaller disc size compared to other devices and this minimizes the risk of aortic regurgitation and embarrassment of coronary artery flow.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.