Anomalous origin of the left coronary artery from the pulmonary trunk is a rare congenital anomaly, first described by Brooks in 1882. It occurs in 0.26% of children with congenital heart disease,Reference Kirklin and Barratt-Boyes1 giving an incidence of 1 in 300,000 live births.Reference Askenazi and Nadas2 The first clinical description was by Bland, Garland and White in 1933.Reference Bland, White and Garland3 Children usually present in infancy with congestive cardiac failure, due to myocardial ischaemia. If the condition is not diagnosed and treated early it is life-threatening with mortality between two-thirdsReference Wesselhoeft, Fawcett and Johnson4 and nine-tenths in the first year of life.Reference Vouhe, Baillot-Vernant and Trinquet5
Whilst the findings from the initial autopsy reported by Brooks suggested there was retrograde flow in the anomalous left coronary artery, EdwardsReference Edwards6 emphasized the dynamic nature of the pathophysiology produced by the lesion. At birth, pulmonary vascular resistance is relatively high, leading to antegrade flow from the pulmonary trunk. Following birth, there is a physiologic decrease in pulmonary vascular resistance and pressure, which results in a critical reduction in perfusion of the left ventricular wall supplied by the anomalous coronary artery. Eventually there is reversal of flow and left-to-right shunting into the pulmonary trunk.Reference Sabiston, Neill and Taussig7
Adequate left ventricular myocardial perfusion, and indeed survival without operative intervention, is dependent on the development of collateral supply from the right coronary artery. Inadequate collateral flow results in left ventricular infarction. This ranges in extent from sub-endocardial to transmural, often causing ischaemic damage to the papillary muscles, and resulting in varying degrees of progressive mitral regurgitation. The rapidity of this sequence has led to the classification of patients into those who present in infancy and those who present in adulthood.Reference Dodge-Khatami, Mavroudis and Backer8
When seen during infancy, there is little or no development of collateral arteries. This leads to the early onset of severe myocardial ischaemia, left ventricular dysfunction and mitral regurgitation, and without operative intervention death results within months of birth. In about one-tenth of patients, however, a sufficient supply of collateral flow develops to permit survival to adulthood. In this subset of individuals, nonetheless, there is an estimated risk of sudden death of between 80% and 90% at a mean age of 35.Reference Alexi-Meskishvili, Berger, Weng, Lange and Hetzer9, Reference Moodie, Fyfe and Gill10
The anomaly is readily corrected by surgery, the aim of which should be reperfusion of the left coronary artery and myocardial salvage. Among the variety of available surgical techniques, transfer of the anomalous left coronary artery to the aorta is the ideal method for long-term patency and adequate blood supply. Creating a tension-free anastomosis can be difficult to achieve. In this report, we describe our experience with 4 different techniques used to treat 12 patients, and highlight our excellent outcomes in the medium term.
Material and methods
All paediatric open-heart operations performed at the Freeman Hospital from 1992 to 2007 were retrieved through coding for anomalous left coronary artery from the pulmonary trunk. We retrieved details of 15 patients, of whom one was referred post-operatively from another centre, 2 had been corrected by means of creation of an aortopulmonary window, while the anomalous left coronary artery had been ligated in another patient. The anomalous artery had been re-implanted to the aorta in the other 11, and these, along with the postoperative patient from another centre, formed the studied cohort.
The clinical records were reviewed to document demographics, presentation, preoperative state, operative details, and postoperative course. Preoperative left ventricular function and mitral valvar competence were assessed by echocardiography. Left ventricular function was measured by determining the shortening fraction. Mitral regurgitation was evaluated and classified as none, mild, moderate or severe. The most recent postoperative echocardiographic data was reviewed by one of the authors. The details are presented in Table 1.
Table 1 Perioperative variables of 12 patients with anomalous left coronary artery from the pulmonary trunk treated with coronary arterial transfer.

Results
Of the 12 patients, 5 had come to attention due to the incidental finding of cardiomegaly on the chest radiograph while being treated for a chest infection. Echocardiography demonstrated a dilated poorly functioning left ventricle. In 3, investigations had been undertaken for an asymptomatic murmur of mitral regurgitation. These were also found to have a poorly functioning ventricle with moderate-to-severe mitral regurgitation. In 3 more cases, the patients had been referred for cardiac transplantation for presumed severe dilated cardiomyopathy, with fractional shortening of less than 10% and moderate mitral regurgitation. One of these children had suffered an out-of-hospital cardiac arrest with successful resuscitation, and another had already undergone corrective surgery with aortic implantation, but continued to be in severe cardiac failure, despite an attempt at balloon dilation of the stenosed left coronary artery. The final patient was referred at the age of 3 months with respiratory distress and failure to thrive (Table 1).
Trans-thoracic echocardiography in all cases demonstrated the anomalous origin of the left coronary artery, and permitted an accurate assessment of left ventricular function and the degree of mitral regurgitation. In our earlier experience, cardiac catheterization was used to confirm the diagnosis, but as we gained experience, and echo – Doppler machines became more sophisticated, we have not needed to perform catheter studies. In one patient, we were unsure of the diagnosis, although the transthoracic echocardiogram had been highly suggestive. In that case, we used transoesophageal echocardiography during the surgical repair to confirm the diagnosis.
Operative technique
All patients were approached through a median sternotomy, with partial thymectomy and pericardiotomy. The finding of large tortuous collaterals from the right coronary artery running across the surface of the right ventricle to the left coronary was highly suggestive of the correct diagnosis.
After dissecting the aorta and pulmonary arteries, and creating slings around the right and left pulmonary arteries, heparin was given and aortic and right atrial cannulation performed for establishing cardiopulmonary bypass. In our earlier cases, we used deep hypothermic circulatory arrest with cardioplegia, but with increasing experience we opted for moderate hypothermia of 28°C. After establishing bypass, cooling was initiated. Once the temperature reached 25°C, we applied an aortic cross-clamp, occluded the pulmonary arteries with the slings, and used 30 ml/kg of antegrade cold blood cardioplegia to create diastolic arrest and enhance myocardial preservation. The cardioplegia was repeated at half dosage after every 30 minutes. The anomalous left coronary artery was then dissected free from the pulmonary trunk, and its mobility assessed. When it reached the aorta without tension, and without any danger of compression, we implanted it directly to the aorta (Fig. 1).

Figure 1 In this technique, the first step (a) is to transect the pulmonary trunk, and harvest the button containing the anomalous left coronary artery. The button is then re-implanted to the aorta (b), and the defect in the proximal part of the pulmonary trunk is repaired using a patch of autologous pericardium.
If it was felt that the coronary artery would be in danger of falling short of the anastomotic site, causing compromise of the arterial blood supply, then we used an alternative technique to create a tension-free anastomosis (Table 1).
Flaps
When the anomalous left coronary artery reaches the aorta but falls short of a length caudally, it is possible to create medially based flaps (Fig. 2), In one of our cases, in spite of using a caudally based flap, a patch of autologous pericardium was also required as a hood to complete the anastomosis (Fig. 3).

Figure 2 This technique creates an inferiorly, or caudally, based flap (a) to accommodate for the distance between the anomalous left coronary artery and the aorta. A patch of autologous pericardium (b) is used to complete the caudally based anastomosis between the anomalous left coronary and the aortic flap. The re-implantation of the anomalous left coronary artery is completed (c) using a patch of autologous pericardium as a hood.

Figure 3 In this technique, a laterally based flap (a) will form a lateral extension for the laterally placed anomalous origin of the left coronary artery from the pulmonary trunk. Note that the artery needs to be harvested on a long button to accommodate for the lack of length. The anomalous artery is then anastomosed (b) to the laterally based flap. The anastomosis is then completed (c), leaving the newly implanted left coronary artery lateral to the aorta.
Extended flap
In other cases, the anomalous left coronary artery falls short of the aorta and direct re-implantation is not possible without tension. It is then possible to create flaps from autologous aorta and the pulmonary trunk so as to provide enough length to produce a satisfactory anastomosis (Fig. 4).Reference Amanullah, Hamilton and Hasan11
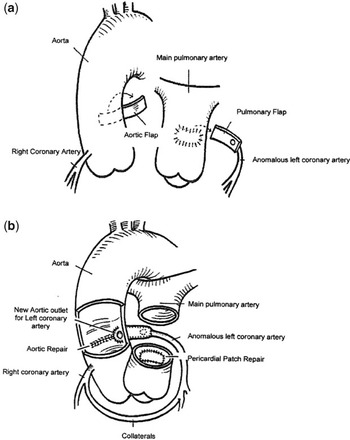
Figure 4 In this technique, flaps from both the aorta and the pulmonary trunk (a) are created to extend the anastomosis of the anomalous left coronary artery. The new opening of the left coronary artery (b) is shown in its appropriate anatomical position, with the aortic flap forming the anterior half and the pulmonary arterial wall completing the main stem of the artery. Pericardium is used to close the defect in the pulmonary trunk, the aorta being closed primarily.
Patch arterioplasty
Occasionally, the first attempt at correction by direct anastomosis may lead to tension at the anastomotic site, or the artery may be compressed as it traverses behind the aorta. We were referred such a case for the possibility of heart transplant. We reassessed the patient and opted for corrective surgery, since the re-implanted anomalous artery was compressed by the aorta over a long segment. We opened the anterior wall of the artery along its entire length, and used the azygos vein as an on-lay patch to increase its size (Fig. 5).

Figure 5 In this technique, the previously implanted anomalous left coronary artery has been repaired using a patch of the azygos vein as an on-lay arterioplasty.
Postoperative outcome
There were no deaths in our series. Chest infections developed in 4 patients with a varying degree of pulmonary sepsis. A right-sided cerebrovascular attack was suffered by 1 patient, which gradually improved before discharge. The median period of postoperative ventilation was 96 hours, with circulatory support needed in 4 patients, 3 with an intra-aortic balloon pump and one with extracorporeal membrane oxygenation. The median stay in intensive care was 7 days, and the median stay in hospital was 13 days, with a range from 7 to 64 days. All patients were discharged on aspirin at 5 mg/kg/day for 3 to 6 months, and captopril at 2–4 mg/kg/day until follow-up. Medication was then titrated according to symptoms or left heart function on echocardiography.
Follow-up has ranged from 5 months to 14 years. On the last presentation to the out-patient clinic, 11 patients were asymptomatic in the first class of the categorization of the New York Heart Association, the remaining patient being in the second class. Captopril was still required by 6 patients. Echocardiography demonstrated normal left ventricular function in 10 patients, with 5 of these having no evidence of mitral regurgitation, and the other 5 having mild regurgitation. The remaining patient had moderate left ventricular function and moderate mitral regurgitation. In some cases, the degree of regurgitation improved over the years of follow-up. None required re-operation, and all patients of school age were attending school regularly.
Discussion
Whilst anomalous left coronary artery from the pulmonary trunk is a rare malformation, it is one of the commonest causes of myocardial ischaemia and infarction in childhood. Untreated, it has a mortality of up to 90% in the first year of life.Reference Vouhe, Baillot-Vernant and Trinquet5 The severe myocardial depression associated with the malformation is unique in that it has no response to medical or interventional therapy. Left ventricular dilation and papillary muscular dysfunction are responsible for mitral regurgitation in the setting of an otherwise structurally normal mitral valve.Reference Jonas12 There may be fibrosis and scarring of the left ventricle depending on the age of the patient, the degree of dominance of the left coronary artery, and the extent of formation of collateral circulation.
A high index of suspicion for diagnosis is required during the work-up of any infant or child with global myocardial ischaemia. The most important differential diagnosis is with dilated cardiomyopathy, which also presents with moderate to severe congestive heart failure, a murmur of mitral insufficiency, and cardiomegaly on plain chest radiography. Despite the availability of a variety of modern imaging techniques, such as computed tomography and magnetic resonance angiography,Reference Khanna, Torigian, Ferrari, Bross and Rosen13 a diagnosis can be readily achieved with an electrocardiogram and cross-sectional echocardiography.Reference Chang and Allada14 If there is uncertainty, coronary angiography may then be indicated. Myocardial viability studies have been used in the assessment of hibernating myocardium, but are not required routinely as they seldom change the surgical approach.Reference Dodge-Khatami, Mavroudis and Backer8
In the current era, establishment of a two coronary arterial system is considered the goal for repair. The establishment of such an arterial system has been shown to result in complete recovery from severe left ventricular dysfunction. Increasing experience with manipulation of neonatal coronary arteries, as part of the arterial switch operation, has led to the current popularity of direct aortic reimplantation.
Early attempts at treatment included simple ligation of the anomalous artery.Reference Sabiston, Neill and Taussig7 Whilst often successful, this is associated with significant mortality and late complications such as the presence of a residual shunt and severe mitral regurgitation.Reference Bunton, Jonas, Lang, Rein and Castaneda15, Reference Backer, Stout and Zales16 Many of the deaths are thought to be due to the fact that, after ligation, the heart is dependent on a solitary coronary arterial system. Recovery of left ventricular function and ventricular remodeling is dependent on establishing a dual coronary arterial circulation. Subsequent surgical treatment, therefore, has concentrated on achieving this goal.
A variety of bypass procedures have been employed, using Dacron interposition grafts, or the long saphenous vein placed from the aorta to the anterior interventricular artery.Reference Cooley, Hallman and Bloodwell17 These conduits are susceptible to thrombosis and occlusion,Reference Backer, Stout and Zales16 especially when prosthetic material is used. The left subclavian artery has also been used to create a dual arterial system.Reference Meyer, Stefanik, Stiles, Lindesmith and Jones18 In the early years of surgical correction, this was particularly attractive, as it could be performed without cardiopulmonary bypass. Kinking of the subclavian artery, and subclavian-coronary arterial anastomotic stenoses, have been reported.Reference Vigneswaran, Campbell, Pappas, Wiggins, Wolfe and Clarke19 Whilst this technique has fallen out of favour, it has still yielded excellent long term results.Reference Lange, Vogt and Horer20
Neches and colleagues were the first to describe the technique of direct implantation.Reference Neches, Mathews and Park21 In some cases, nonetheless, translocation of the artery was not thought to be possible due anatomical constraints. In 1979, Takeuchi and colleagues described an alternative technique, creating a tunnel from the aorta to the orifice of the anomalous left coronary artery via an aorto-pulmonary window using pulmonary arterial wall.Reference Takeuchi, Imamura and Katsumoto22 A high origin of the right coronary artery, or location of the left coronary artery close to a pulmonary valvar leaflet, may lead to complications such as baffle thrombosis or supravalvar stenosis.Reference Smith, Arnold and Anderson23
Katsumata and Westaby have described an alternative technique of transferring a low, lateral anomalous left coronary from the left posterior pulmonary sinus to the aorta.Reference Katsumata and Westaby24 They used native aortic and pulmonary arterial wall to create an extension of the left coronary artery in the vertical plane.
SeseReference Sese and Imoto25 and van SonReference van Son and Mohr26 have described a trapdoor technique using autogenous aortic and pulmonary arterial walls to create an extension of the anomalous left coronary artery. The use of combined aortic and pulmonary tissue is thought to be preferable to the use of pulmonary tissue alone because it is possible to fashion a wider neo-coronary orifice.Reference Turley, Szarnicki, Flachsbart, Richter, Popper and Tarnoff27 The trapdoor technique extends the left coronary artery in the horizontal plane. In the description by Sese, the anomalous left coronary arose from the left anterior aspect of the pulmonary trunk, and therefore the neo-left coronary artery lay anterior to the pulmonary trunk, whereas in the patient described by van Son, the left coronary artery took origin from the left posterior pulmonary sinus and thus the left coronary artery was routed posterior to the pulmonary trunk. Although both techniques produced good results with improved myocardial perfusion, left ventricular function and a reduction in mitral regurgitation, Black et al have reported that a translocated anomalous left coronary lying anterior to the pulmonary trunk is more prone to compression during periods of increased cardiac output.Reference Black, McCrindle and Freedom28 Our technique of using extended flaps,Reference Amanullah, Hamilton and Hasan11 therefore, has the advantage of the neo-left coronary artery lying in a horizontal and anatomically correct axis, running in the anatomical groove behind the pulmonary trunk.
We have summarized the normal and the anomalous sites of origin of the left coronary artery from the pulmonary trunk in our cases in Figure 6. When the anomalous left coronary artery originates from the rightward aspect of the posterior facing sinus, it can usually be transferred without tension to the posterior-lateral wall of the aorta. If the anomalous left coronary originates from the leftward aspect of the posterior facing sinus, simple transfer may leave the anastomosis under tension, and in these cases use of inferior or medially based flaps can allow tension-free anastomosis. When the anomalous left coronary originates from the non-facing sinus, then extended flaps must be created to provide a tension-free anastomosis.

Figure 6 Panel a shows the normal relationships of the aorta and pulmonary trunk, and the usual origins of coronary arteries. In Panel b, we illustrate an anomalous left coronary artery arising from the medial aspect of the left facing sinus, while Panel c shows an anomalous left coronary artery arising from the lateral aspect of the left facing sinus. Panel d shows the anomalous left coronary artery arising from the non-facing sinus, which is very rare.
One of the other major considerations in treatment is the approach to the mitral valve. Some investigators recommend routine mitral annuloplasty on the grounds that early postoperative cardiac output is improved. Other authors recommend a slightly more conservative approach with repairReference Alexi-Meskishvili, Berger, Weng, Lange and Hetzer9 or replacementReference Bunton, Jonas, Lang, Rein and Castaneda15 of the mitral valve in cases of severe mitral regurgitation. Our experience, and that of several other groups,Reference Dodge-Khatami, Mavroudis and Backer8, Reference Lange, Vogt and Horer20, Reference Turley, Szarnicki, Flachsbart, Richter, Popper and Tarnoff27 shows that mitral reconstruction does not need to be addressed at initial operation. The added ischaemic time in the setting of severely compromised ventricular function is potentially more harmful.
The reported perioperative mortality for reimplantation of the anomalous left coronary into the aorta is between 0% and 16%.Reference Lange, Vogt and Horer20, Reference Cochrane, Coleman, Davis, Brizard, Wolfe and Karl29, Reference Vouhe, Tamisier and Sidi30 Preoperative risk factors for mortality are poor preoperative left ventricular function and young age at operation. Whilst some investigatorsReference Schwartz, Jonas and Colan31 have suggested that severe preoperative mitral regurgitation is a risk factor this has not been conclusively proven.Reference Lange, Vogt and Horer20 The favourable postoperative outcome in our cohort of patients may be explained, at least in part, by the fact that the majority of patients did not present with classical symptoms and signs of congestive heart failure, and only 4 patients had a fractional shortening of less than 10%.
Conclusion
No matter how severe the ventricular dysfunction appears, we recommend aortic implantation as the primary therapy because functional recovery of the left ventricle and the mitral valve can be expected. Our experience suggests that mitral valve reconstruction is not needed at the time of initial repair. Flaps of autogenous tissue may be required to fashion a tension-free anastomosis.









