Congenital coronary artery anomalies are cardiac lesions associated with deviations from typical cardiac artery origin, course, and number.Reference Kastellanos, Aznaouridis, Vlachopoulos, Tsiamis, Oikonomou and Tousoulis1 In typical coronary anatomy, the right coronary artery arises from the anterior aortic right sinus of Valsalva and the anterior left coronary artery arises from the aortic left sinus of Valsalva.Reference Tomanek and Angelini2 The incidence of coronary artery anomalies varies between diagnostic modality and population studied but is best estimated between 0.17 and 1.2%.Reference Graidis, Dimitriadis and Karasavvidis3 Although most congenital coronary artery anomalies are clinically benign, some are associated with cardiac ischemia, arrhythmias, and heart failure and collectively contribute to the second most common cause of sudden cardiac death in children.Reference Ackerman, Atkins and Triedman4
Anomalous origin of a coronary artery from the pulmonary artery is a rare subtype of malignant coronary artery anomaly with four proposed variations: 1) anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA), 2) anomalous origin of the right coronary artery from the pulmonary artery (ARCAPA), 3) anomalous origin of a branch vessel (circumflex or left anterior descending artery) from the pulmonary artery (ACxAPA and ALADAPA, respectively), and 4) total anomalous origin of the coronary arteries from the pulmonary artery (TCAPA).Reference Soloff5 Of these variants, ALCAPA is the most common with an estimated incidence of 0.021%.Reference Werner, Wróblewska-Kałuzewska, Pleskot, Tarnowska and Potocka6 In this lesion, the left coronary artery is supplied with oxygenated blood from collaterals of the right coronary artery. This creates a functional “coronary steal” as blood flows retrograde in the left coronary artery and eventually into the pulmonary artery.Reference Chattranukulchai, Namchaisiri and Tumkosit7 Patients can present early in life with evidence of ischemia from inadequate collateralisation or later in life from: 1) disruption in blood flow in the single-aortic-derived coronary artery system or 2) development of cardiac dysfunction from long-standing coronary steal.Reference Yau, Singh, Halpern and Fischman8 The degree of ischemia and ventricular dysfunction is usually correlated with the degree of collateralisation from the contralateral coronary artery as well as pulmonary artery pressure. Treatment is aimed at establishing an aortic-derived two-vessel coronary artery system and eliminating the associated coronary steal.Reference Boutsikou, Shore and Li9
TCAPA is a rare coronary anomaly with unknown incidence. Semantically, this lesion differs from the coronary circulation seen in transposition of the great arteries, whereby the coronaries originate from the anatomic aorta. In TCAPA, the entire coronary artery circulation originates from the pulmonary artery, not allowing compensatory mechanisms like collateralisation to supply oxygenated blood to the territory supplied by the anomalous circulation. Once touted as a coronary anomaly that was “uniformly fatal” and with “little clinical interest with death occurring before serious study can be undertaken,” TCAPA is becoming better recognised given increased awareness and improved diagnostic modalities.Reference Blake, Manion, Mattingly and Baroldi10,Reference Colmers and Siderides11 Multiple case reports of TCAPA have been published with variable presentation from cyanosis in a newborn boy to an asymptomatic murmur in an otherwise healthy 7-year-old girl.Reference Feldt, Ongley and Titus12,Reference Monselise, Vlodaver and Neufeld13 The aim of this review was to better characterise the presentation, utilised diagnostic modalities, associated cardiac lesions, and employed treatment strategies in patients with TCAPA to gain a better understanding of this rare coronary artery anomaly. This will in turn serve as a consolidated resource for clinicians that encounter this lesion as well as provide a deeper understanding of coronary artery anomalies and disease. Furthermore, this review is meant to increase the index of suspicion of this lesion and prompt earlier workup and diagnosis.
Material and method
Search strategy
A systematic review was performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology for cases of TCAPA using PubMed, Embase, and Web of Science.Reference Moher, Liberati, Tetzlaff and Altman14 Keywords searched included “total anomalous origin of the coronary arteries from the pulmonary artery,” “single ostium anomalous coronary artery from the pulmonary artery,” and “anomalous origin of both coronary arteries from the pulmonary artery.” The search took place in January 2020.
Inclusion criteria
To be included for collective analysis, it was necessary for the published report to describe a case where both the right coronary artery and left coronary artery originated from the pulmonary artery, either as a single coronary artery or as separate branches. Additionally, the age of the patient, as well as some aspect of clinical presentation, was necessary for inclusion. Furthermore, it was necessary for the case report, or at least the abstract where pertinent information could be gathered, to be published in English or Spanish. Data regarding physical exam findings, method of diagnosis, cardiac catheterisation data, associated cardiac lesions, anatomic origin/course of the anomalous vessel, treatment strategy, pathologic assessment, and outcomes were gathered when available.
Data collection
Cases were screened and assessed for eligibility by two independent reviewers (TG and SC) and discrepancies were resolved by consensus. Data were collected from each eligible manuscript by one reviewer (TG) and tabulated into a Microsoft Excel spreadsheet.
Statistical analysis
Quantitative data were displayed as means with standard deviations or medians with a range and analysed using a student t-test when appropriate. Qualitative data were displayed as frequencies and analysed using a chi-square test when appropriate. Statistical significance was determined by a p-value < 0.05.
Results
On primary search, 1014 manuscripts were identified (PubMed 580, Embase 248, and Web of Science 186) (Fig 1). After duplicates were removed and the manuscripts were screened and assessed for eligibility, 50 manuscripts corresponding to 57 cases were selected for inclusion (Supplementary File S1). The median year of publication was 1988 (mean 1988, range 1931–2020) (Fig 2). There were 50 cases with gender noted with a slight male predominance (n = 29, 58%). The median age at presentation was 10 days (mean 1.71 ± 6.6 years, range 0 days to 39 years) (Fig 3). The median age at presentation for male patients was 15.0 days, (mean 2.25 ± 8.39 years, range 0–39 years) and for female patients the median was 7.3 days, (mean 1.52 ± 5.27 years, range 0–23 years) (p-value 0.72). In 28 cases, the birth weight of the patient was listed with a median of 2.97 kg (mean 3.05 ± 0.59 kg, range 1.81–4.42 kg). Maternal age was listed in 11 cases and the median age was 26 years (mean 25.8 ± 4.26 years, range 19–34 years). Cases were most commonly published from North America (n = 31, 54.4%), but cases were included from Europe (n = 12, 21.1%), Asia (n = 12, 21.1%), and Australia (n = 2, 3.5%).

Figure 1. Systematic review search strategy using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology.

Figure 2. Distribution of year of publication in patients with TCAPA.

Figure 3. Distribution of patients with TCAPA based on age.
Cyanosis and respiratory distress were the most common presenting symptoms (n = 22, 38.6% and n = 14, 24.6%, respectively) (Table 1). Interestingly, one patient was asymptomatic at the time of diagnosis and was evaluated for a murmur. In 21 patients, a murmur was heard on physical exam (36.8%). Six patients had undergone a congenital heart surgery/procedure prior to the diagnosis of TCAPA; repair of coarctation of the aorta (n = 3), ventricular septal defect closure (n = 1), subclavian to pulmonary artery shunt (n = 1), and pulmonary artery banding (n = 1). Not surprisingly, diagnosis was most commonly made at autopsy (n = 26, 45.6%), but cases were also diagnosed by angiography (n = 16, 28.1%), at the time of surgery (n = 9, 15.8%), echocardiography (n = 3, 5.3%), cardiac CT (n = 1, 1.8%), and unlisted (n = 2, 3.5%). The median year of publication in patients who were diagnosed at autopsy was 1970, angiography 2001, cardiac CT 2012, at the time of surgery 2014, and echocardiography 2015.
Table 1. Distribution of symptoms in symptomatic patients (non-mutually exclusive)

Mitral regurgitation was the most common valvular abnormality described on echocardiography, seen in nine patients. A pre-operative assessment of ejection fraction was performed in eight patients with a mean of 24.8%. An associated cardiac lesion (determined via echocardiography or at the time of surgery/autopsy) was present in 44 cases (77.2%), with a ventricular septal defect or patent ductus arteriosus representing the most common lesions (n = 20 and 18, respectively) (Table 2). The majority of cases (66.7%, n = 38) were associated with a single-orifice coronary artery and the remainder associated with dual-orifice coronary arteries (n = 19). The origin/course of the anomalous coronary artery/arteries was described in 49 cases (86.0%) (Fig 4). In cases where a pathologic assessment of the myocardium was performed, 14 (60.9%) were associated with evidence of ischemia on histology.
Table 2. Associated cardiac lesions with more than one occurrence seen in TCAPA (non-mutually exclusive)

RV: right ventricle; R: right; L: left.
Table 3. Management strategies utilised in 31 patients with TCAPA not diagnosed at autopsy

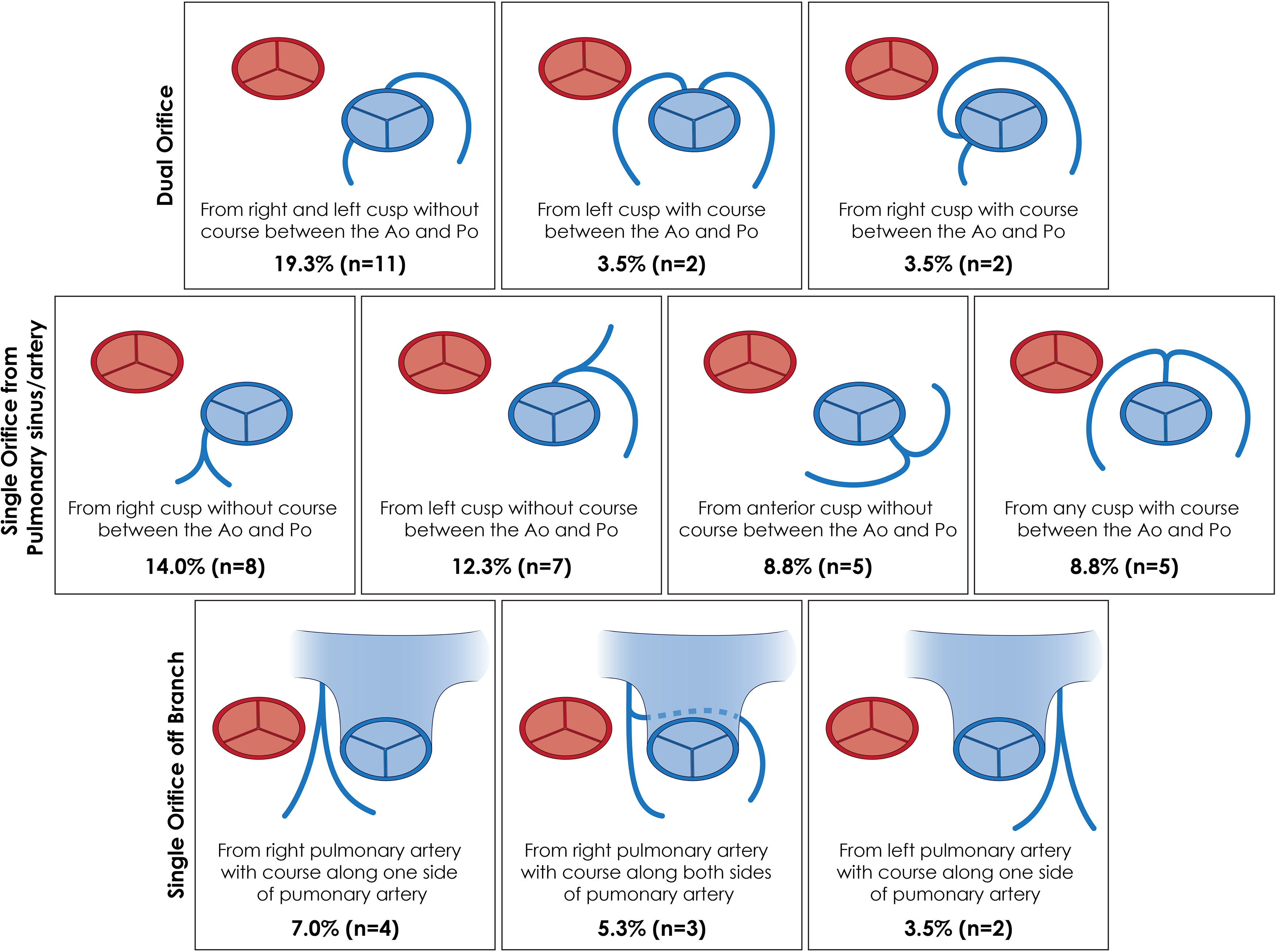
Figure 4. Origin and course of the anomalous coronary artery in 49 patients with TCAPA described in this review. (Ao: aorta, Po: pulmonary artery).
Operative intervention was pursued in 26 cases (45.6%) (Table 3). Treatment modalities that were utilised more than once included aortic re-implantation of the anomalous vessel/vessels (n = 14) and a Takeuchi-type repair (n = 7). The median age of patients who underwent aortic re-implantation was 60 days and the median age of patients undergoing a Takeuchi repair was 61 days (p-value 0.596). The median year of publication in cases that utilised the Takeuchi method of repair was 1987 and for aortic re-implantation was 2015. A mortality rate of 46.2% was observed in all patients who underwent operative intervention; 28.6% with aortic re-implantation and 71.4% with the Takeuchi repair. In those who died after operative intervention, the median post-operative day (POD) of death was 0.5 (mean POD 5.33). The median day of discharge was POD 14 (mean POD 17), which was listed in eight cases. The mean follow-up time was 180 days (mean 225 days), which was reported in 11 cases. Twelve cases reported outcomes after operative intervention; improvements in symptoms were seen in 3 cases and improvements in objective assessments of cardiac function (stress test, myocardial perfusion, or echocardiography) in 11 cases.
Discussion
Coronary artery development is a poorly understood phenomenon with tremendous clinical implications. In the third/fourth week of gestation, as the myocardium thickens, a vascular plexus without connection to the aorta forms and develops into an immature coronary artery.Reference Angelini15 The exact mechanism of establishing connection of the immature coronary artery to the aorta is unknown, but thought to be related to “in-growth” of the immature coronary artery with signalling from neural crest cells, vascular endothelial growth factor, and chemokine CXCL12.Reference de Oliveira Silva-Junior, da Silva Miranda and Mandarim-de-Lacerda16,Reference Ramai, Lai, Monzidelis and Reddy17 Disruptions in those pathways, whether genetic or environmental, likely contribute to the formation of coronary artery anomalies and anomalous origin of a coronary artery from the pulmonary artery.Reference Pérez-Pomares, de la Pompa and Franco18 Review of associated lesions seen in TCAPA may shed some light into the pathways associated with its development. Although our sample size was low, bicuspid aortic valve was seen in 8.8% of patients in this cohort, which differs from the 0.5–2% incidence found in the general population.Reference Tutar, Ekici, Atalay and Nacar19 Associations between bicuspid aortic valve and anomalous origin of a coronary artery from the pulmonary artery have been described in animal studies as well as in ACxAPA/ALADAPA and again may hint towards a common embryologic pathway.Reference Fernández, Durán and Real20 One interesting case identified in this review was a patient diagnosed with TCAPA at autopsy who had a sibling diagnosed with ALCAPA.Reference Goldblatt, Adams, Ross, Savage and Morris21 Though it is possible that the association of these two rare diagnoses was a mere coincidence, this observation supports, but does not demonstrate, a genetic or environmental basis for anomalous origin of a coronary artery from the pulmonary artery.
An understanding of the direction of blood flow in anomalous origin of a coronary artery from the pulmonary artery is necessary in understanding the associated pathophysiology. In 1886, John Brooks hypothesised the direction of blood flow in the anomalous vessel to be retrograde based on pathologic review of a patient with ARCAPA.Reference Brooks22 This theory remained unsupported until 1960 when Drs. David Sabiston Jr, Catherine Neill, and Helen Taussig published their findings regarding a 2.5-month-old boy with ALCAPA.Reference Sabiston, Neill and Taussig23 They sampled blood from the left coronary artery and pulmonary artery and found the oxygen saturation in the left coronary artery to be 100% compared to 76% in the pulmonary artery, thereby confirming retrograde flow. It is now known, based on detailed angiographic/echocardiographic findings in ALCAPA/ARCAPA, that antegrade flow of deoxygenated blood occurs in the anomalous vessel within the first 2 weeks of life when pulmonary resistance is high.Reference Singh, Di Carli, Sullivan, Leonen and Morrow24 Once pulmonary resistance decreases and collateralisation with the aortic-derived coronary artery is developed, retrograde flow of blood ensues and becomes reliant on the higher pressure from the aortic-derived coronary artery. However, in TCAPA, an aortic-derived coronary artery is not present and antegrade flow of deoxygenated blood continues after pulmonary resistance decreases.Reference Abdelmohsen, Alkhushi, Bahaidarah, Shihata and Jamjoom25 In this scenario, coronary perfusion pressure is directly related to pulmonary artery pressure. Cardiac lesions that increase pulmonary artery pressure such as ventricular septal defect, aorto-pulmonary window, and truncus arteriosus would therefore augment coronary perfusion pressure. Alternatively, lesions associated with left to right shunting (such as a ventricular septal defect or patent ductus arteriosus) would additionally increase the mixed venous oxygen saturation and would theoretically augment coronary artery oxygen saturation and in this cohort were associated with later ages of presentation in patients with concomitant TCAPA.
The clinical presentation of patients with TCAPA was found to be quite variable. Pulmonary artery pressure decreases after birth as intrinsic pulmonary resistance decreases as the lung expands and the ductus arteriosus closes.Reference Teitel, Iwamoto and Rudolph26 This change is most dramatic in the first 2–3 days of life and adult range pulmonary artery pressures are achieved by 2 weeks of life.Reference Rudolph27 In TCAPA, this decrease in pulmonary artery pressure (therefore coronary perfusion pressure) is maladaptive and likely contributes to the cohort of patients presented within the first 2 weeks of life. Observations of extensive myocardial hypertrophy/remodelling, fibrosis, and other pathologic evidence of chronic ischemia have been seen at autopsy in some patients with TCAPA immediately after birth and are suggestive of prenatal insults.Reference Swann and Werthammer28 In normal fetal anatomy, a flap of tissue known as the Eustachian valve preferentially directs the flow of well-oxygenated blood from the inferior vena cava/placenta towards the foramen ovale and into the left atrium/ventricle, thereby preferentially delivering a relatively high concentration of oxygenated blood to the heart/brain. Alternatively, desaturated blood from the superior vena cava is preferentially directed towards the tricuspid valve and then away from the pulmonary circulation into the proximal descending aorta via the ductus arteriosus.Reference Murphy29 For this reason, relatively decreased coronary oxygen saturations in patients with TCAPA in utero may theoretically lead to cardiac dysfunction prior to delivery and thus explain the chronic appearing pathologic changes observed in some patients soon after birth.
Although only half of patients with TCAPA presented within the first 2 weeks of life, 91.2% of all patients became symptomatic within the first year of life. Reasons for the delay in presentation can most likely be attributed to the presence of associated cardiac lesions that maintained increased pulmonary pressure and mixed systemic venous oxygen saturation such as ventricular septal defect, patent ductus arteriosus, aorto-pulmonary window, and truncus arteriosus. The presence of associated cardiac lesions causing a right to left shunt is one explanation for the relatively high rate of cyanosis observed in patients with TCAPA. However, this explanation is not complete, as three patients with TCAPA and no other cardiac lesions presented with cyanosis. This might be explained by the myocardial ischemia associated with TCAPA leading to heart failure, as echoed by the observed decreased ejection fraction seen in this cohort of patients with TCAPA. The cohort that presented beyond 2 weeks of life without an associated cardiac lesion might be explained by the fact that as left ventricular function deteriorates due to ischemia, diastolic dysfunction ensues resulting in elevated left atrial pressure (leading to pulmonary hypertension) that then would augment flow to the anomalous coronary arteries.
Two-thirds of patients with TCAPA were associated with a single coronary artery arising from the pulmonary artery. Single coronary artery from the aorta is a well-described rare lesion with an estimated incidence between 0.024 and 0.066%.Reference Desmet, Vanhaecke and Vrolix30,Reference Lipton, Barry, Obrez, Silverman and Wexler31 Patients can present incidentally or with evidence of coronary ischemia. Depending on the course of the anomalous single coronary artery from the aorta, treatment is typically reserved for symptomatic patients.Reference Akcay, Tuncer and Batyraliev32 A classification system was described by Lipton et al. and then modified by Yamanaka et al. who detailed the origin and course of the single coronary artery from the aorta.Reference Lipton, Barry, Obrez, Silverman and Wexler31,Reference Yamanaka and Hobbs33 This classification system is not applicable to TCAPA, as components of this classification system describe the origin from the aortic sinus of Valsalva as well as branching from “normally” located coronary arteries. The characterisation in Figure 4 better represents not only the number of coronary arteries involved in TCAPA, but also the phenotypic distribution of their observed origin and course as it relates to the pulmonary artery.
Although the diagnosis of TCAPA was made at autopsy in 46% of patients, improvements in echocardiography, angiography, and cross-sectional imaging have led to an increased number of premortem diagnoses and the potential for operative intervention. Failure to obtain a pre-operative diagnosis was associated with death for two reasons: 1) failure to achieve adequate myocardial protection during administration of cardioplegiaReference Vitanova, Cleuziou, Deutsch, Ackermann and Schreiber34 and 2) correction of a cardiac lesion that then decreased pulmonary pressure, thereby unknowingly disrupting coronary perfusion.Reference Feldt, Ongley and Titus12 Angiography or CT angiography offers the advantage of identifying the origin/course of the anomalous vessel and should be considered the gold standard for diagnosis. The inability to identify coronary ostia or coronary flow on left-sided angiography prompted investigation via right-sided angiography and led to an angiographic diagnosis in 16 patients with TCAPA. Echocardiography has emerged as a non-invasive method to diagnose many coronary artery anomalies.Reference Angelini35 Assessments of ostial location and direction of flow through the anomalous vessel on echocardiogram can help differentiate variants of anomalous origin of a coronary artery from the pulmonary artery, although their sensitivity and specificity are not perfect. Numerous cases of TCAPA were incorrectly diagnosed as ALCAPA based on pre-operative echocardiogram, likely related to the inherent difficultly assessing the right coronary artery on echocardiography.Reference Zhang, Zhang and Gao36–Reference Donmez, Aykan and Yilmaz38 However, in ALCAPA, flow through the anomalous left coronary artery is more commonly retrograde, whereas in TCAPA flow is antegrade (from the pulmonary artery).Reference Abdelmohsen, Alkhushi, Bahaidarah, Shihata and Jamjoom25 The distinction between TCAPA and ALCAPA in the pre-operative setting is important because the protective effects of collateralisation from an aortic-derived coronary are not present in TCAPA, therefore increasing the urgency of operative intervention. Other echocardiographic findings suggestive of TCAPA, similarly seen in ALCAPA, include depressed left ventricular function and dilation, and often associated mitral regurgitation.Reference Thatte, Kirakosian, Kaza and Friedman39
Treatment of TCAPA is aimed at creating an aortic-derived coronary artery system. Prior to surgical intervention, prostaglandin E1 should be considered in newborns to preserve the ductus arteriosus and augment coronary perfusion pressure and pulmonary artery oxygen saturation.Reference Karimi, Hulsebus and Lutin40 Although isolated percutaneous or surgical ligation of the anomalous vessel at its origin has been described in the treatment of ALCAPA/ARCAPA, this strategy has no role in TCAPA.Reference Collins, Colman, Benson, Hansen, Merchant and Horlick41,Reference Francis and Brown42 This is due to the dependency of the pulmonary artery for blood flow in TCAPA and the absence of coronary steal associated with the lesion. Likewise, ligation of the anomalous vessel at its origin with coronary artery bypass grafting has been a utilised treatment strategy in ALCAPA/ARCAPA. Though this method does establish an aortic-derived coronary artery system, it makes the entire coronary circulation reliant on bypass graft patency; a significant long-term concern given the age of presentation among patients with TCAPA. The two main treatment strategies that were used in TCAPA patients were aortic re-implantation or a Takeuchi repair using an intra-pulmonary baffle. An objective comparison between techniques is not possible in this review given a lack of standardisation, small sample size, and limitation in long-term follow-up/patency. Nevertheless, a greater than twofold increase in mortality was seen with the Takeuchi repair. This could be related to: 1) known complications related to the Takeuchi repair such as baffle leak, damage to the pulmonary valve, or supra-valvular pulmonary artery stenosisReference Ginde, Earing, Bartz, Cava and Tweddell43 or 2) the median year of publication for patients undergoing the Takeuchi repair was 28 years earlier than the median year of publication for aortic re-implantation. Nevertheless, clinicians should proceed with caution if the Takeuchi repair is chosen over aortic re-implantation, given this observation in mortality.
There are numerous limitations to this review. First, there is an inherent bias in the cases that were included in our cohort as all came from published manuscripts. Although TCAPA is in itself rare, there is a push to publish rare presentations and associated lesions that may have altered some of our phenotypic observations. Next, some aspects assessed, such as presenting symptoms, physical exam findings, and outcomes are inherently subjective. Given the lack of standardisation, collective analysis becomes difficult and does introduce bias. Likewise, cases were included from 1931 to 2020. Major improvements in diagnostic modalities, cardiopulmonary bypass, and peri-operative care make collective analysis problematic. Furthermore, the data that were tabulated included only what was written by the author of each manuscript; data from all patients regarding cardiac catheterisation, echocardiography, and pathologic assessment of tissue were not present in each case. Although these could have been made inclusion criteria, they would have significantly decreased the overall sample size. Lastly, although nearly 20% of manuscripts had follow-up data, long-term follow-up is lacking which makes comparison of treatment strategies difficult. Nevertheless, analysis of case reports likely represents the only current avenue for gaining a better understanding of rare lesions such as TCAPA until clinical databases are robust enough to provide a sample size large enough for meaningful analysis.Reference Carey44
Conclusion
TCAPA represents a rare coronary anomaly that is not uniformly fatal as was initially believed. The clinical presentation of patients with TCAPA was found to be variable, likely related to the presence of concomitant cardiac lesions that keep pulmonary pressures elevated such as associated left to right shunts. Two-thirds of cases were associated with single coronary ostia, and both aortic re-implantation and a Takeuchi repair have been utilised in the treatment of TCAPA. Given their inherent similarities, TCAPA should be considered in patients with suspected ALCAPA for the serious consequences that can occur if not promptly corrected. This review offers a deeper understanding of TCAPA for clinicians who might encounter this lesion and provides some further insights into the coronary artery development.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951121002997
Acknowledgements
None
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors
Conflicts of interest
The views expressed in this material are those of the authors and do not reflect the official policy or opinion of the US Government, the Department of Defense, or the Department of the Air Force.
Ethical standards
This research does not involve human and/or animal experimentation.










