Published online by Cambridge University Press: 24 May 2005
We describe a new method of three-dimensional magnetic resonance imaging of the heart that has been used to produce high quality diagnostic images in 274 patients with congenital cardiac disease, ranging in age from 1 day to 66 years. Using a steady state free precession gradient echo technique and parallel imaging, rapid acquisition of the entire cardiac volume is possible during 8 to 15 sequential breath-holds, each lasting between 8 and 15 s. We obtained high-resolution images, with a resolution of 1 mm3, at between 3 and 10 phases of the cardiac cycle.
While images of diagnostic quality were obtained in all cases, in 52 patients there was some degradation due to various factors. Children under 8 years were ventilated, and ventilation was suspended for the breath-holds. For patients breathing spontaneously a novel respiratory navigator technique was developed, using a navigator echo placed over the right hemidiaphragm. This was used successfully in 20 patients, and reduced the misalignment of images obtained during different breath-holds.
Images were analysed using multi-planar reformatting and volume rendering. Image processing took approximately five minutes for each study. End-diastolic images were processed for all patients. Systolic images were also processed in selected cases.
Further improvements in parallel imaging should reduce imaging times further, so that it is possible to obtain the full volume image in a single breath-hold. This will enable imaging of complex anatomy to be obtained using a standard imaging protocol that does not require the operator to understand the cardiac malformation, making the magnetic resonance imaging of congenital cardiac disease faster and more effective.
Management of patients with congenital cardiac disease relies heavily on imaging. Routine imaging techniques, such as echocardiography and X-ray angiography, have been recently complemented by cardiac magnetic resonance,1 which provides the capability of imaging in any plane. This distinguishes it from echocardiography, which is limited by acoustic windows, and X-ray angiography, which only provides projections. Unfortunately, the three-dimensional nature of some congenital cardiac defects is difficult to demonstrate with two-dimensional images. In addition, due to the complex nature of some malformations, the choice of the optimal plane for imaging requires expert knowledge of cardiac anatomy. The requirement of operators with knowledge of both magnetic resonance imaging and congenital heart defects has been a major hindrance to a more widespread use of this technique. The development of three-dimensional techniques for acquisition of images now opens up the possibility of improved visualisation of cardiac anatomy without the requirement for expert planning.
Gadolinium enhanced magnetic resonance angiography is an accurate three-dimensional technique that allows assessment of congenital malformation of the aorta, pulmonary arteries, and veins.2 The lack of cardiac synchronisation, however, leads to blurring of the image due to cardiac motion, which is particularly problematic when assessing intra-cardiac anatomy. We have previously shown the benefit of axial multi-slice gradient echo acquisition in delineating complex anatomy without expert planning.3 This technique enabled slice-by-slice examination and reconstruction, along with visualisation of the cardiac volume. Each slice was acquired over 1 breath-hold, leading to acquisition times of up to 40 min. In addition, to provide sufficent signal, the maximum “through plane” resolution was limited to 6 mm, leading to some loss of resolution and accuracy in the “through plane” direction. This was compounded by differences in breath-hold position between slices. It was therefore necessary to smooth the image to obtain an acceptable quality of rendering, with the concern that the smoothing resulted in loss of important anatomical detail. Rendering also requires time-consuming manual segmentation of every slice. Steady state free precession, a newer gradient echo technique, provides greater contrast between blood and myocardium. Recently, three-dimensional techniques for steady state free precession have been developed that allow fast imaging of the entire cardiac volume. The improved contrast and resolution of this technique also allows simpler reconstruction based on thresholding, rather than the time-consuming manual segmentation required for gradient echo techniques.4 The aim of this study is to show the benefit of this new technique in the imaging of patients with congenital cardiac malformations.
The population consisted of 274 children and adults with congenital heart disease referred to our unit for cardiac magnetic resonance imaging between February 2002 and April 2003 (Table 1). The median age was 22.7 years with a range between 1 day and 66 years. Patients under the age of 8, 103 in number, underwent the procedure under general anaesthetic.
Table 1. Number of patients in each age group.
All imaging was performed on a 1.5 T scanner (Intera, Philips, Best, The Netherlands) and, depending on size, phased-array coils with between two and five elements were used. A vector electocardiographic system was used for cardiac synchronisation.
So as to plan the procedure, a steady state free precession scout scan of the cardiac anatomy lasting 12 s was acquired in the axial, coronal and sagittal planes. This was followed by a 30 s sensitivity encoding reference scan. Sensitivity encoding is a method of speeding up subsequent imaging by a factor of 2. Following the acquisition of half of the image, sensitivity encoding allows reconstruction of the full image by using spatial information from multiple coils.5
The three-dimensional steady state free precession volume scans were acquired using a multi-chunk, multi-breath-hold, multi-phase technique. The repetition time was approximately 4 ms, the echo time was approximately 2 ms, the field of view was between 300 mm and 350 mm, and the size of the image was approximately 192 pixels by 256 pixels. As imaging the whole cardiac volume was not possible in 1 breath-hold, the scan was divided into 8 to 12 smaller chunks. Each chunk was acquired as a single breath-hold of 8–15 s. The acquisition time for the whole sequence was between 6 and 10 min. The scan was acquired in the axial plane and therefore required no complex planning. High quality images were reconstructed from the raw data, with a resolution of approximately 1 mm in all directions. Typically one volume scan yielded 80 to 144 slices, compared to 30 to 40 slices in our previous approach.3
Each volume was also acquired in multiple phases of the cardiac cycle. In most cases, delineation of anatomy was the most important consideration. So as to keep scan times to a minimum, only 3 to 7 phases of the cardiac cycle were acquired. In some cases, 10 phases were obtained, each acquired in approximately one-tenth of the cardiac cycle to give information about cardiac motion and provide sharp systolic images, but with a resulting increase in scan time to 25 to 30 s per breath-hold.
In ventilated patients, breath-holds were achieved through suspension of ventilation. In this way, the position of the heart was uniformly maintained between chunks, preventing misregistration of volume data. Unfortunately, in patients who are awake, sectioning between successive breath-holds is less reproducible. To prevent misalignment of volumes, a novel respiratory navigator technique was developed and used in 20 non-ventilated patients in the latter part of the study. A navigator echo was placed through the right hemidiaphragm, acquiring the position of the diaphragm during the first breath-hold. Images during subsequent breath-holds were only acquired if the diaphragm was within a window of 2.5 mm of the original position. Within this respiratory window, the acquisition volume was moved by a factor of 0.6 of diaphragm excursion to compensate for motion.
In most patients, Gadolinium enhanced three-dimensional magnetic resonance angiograms were also obtained. The repetition time was approximately 5 ms, the echo time was approximately 2 ms, the field of view was between 250 mm and 350 mm, and the image size was approximately 512 pixels by 153 pixels.
Images were analysed offline on a commercial image analysis workstation (EasyVision 5.1, Philips Medical Systems, Best, The Netherlands). As well as looking through the volume in the original axial slices, we used two additional techniques, multi-planar reformatting, which allows viewing of the volume in any plane, and volume rendering. Volume rendering of the blood pool was performed using an automated segmentation technique in which the user sets a threshold and seeds the structures of interest by clicking on them with the mouse. This enables different structures of the heart to be segmented separately, and given different colours in the process of rendering. A rendered image is generated on the same workstation, and can be visualised from any direction, and movies of the rotating heart can be generated for subsequent reporting. In most patients, only the end diastolic images were segmented, but where this was of clinical interest, the systolic volume could also be rendered. The segmentation and rendering process for each patient took between 5 and 10 min. Occasional studies with less myocardial blood pool contrast in the images were more difficult to reconstruct, and scrolling through the volume data set was used as the main method of visualisation.
The procedure was well tolerated, and diagnostic images were obtained in all patients. The time needed for acquisition time was not recorded in all cases, but consideration of a sample of cases showed that the scan times with three dimensional steady state free precession were approximately a quarter of those needed using our previous technique. Our current average was 8 min. The processing time was approximately 5 min, compared to an average processing time of 40 min using our previous method.
In 52 patients, the images had degraded in some slices due to turbulence of blood flow, long echo and repetition times, motion artefact from inability to breath-hold during the scan, and metallic artefact. In most cases, the degradation was mild, being confined to the branches of the pulmonary trunk, with relative sparing of the intra-cardiac anatomy. In two-fifths of patients examined while awake, mild misregistration of volume rendered chunks was present. This misalignment was reduced by using the respiratory navigator in the later cases (Fig. 1).

Figure 1. Reformatted image of the right ventricular outflow tract (a) without respiratory navigator and (b) with respiratory navigator.
We found the images particular valuable in more complex intra-cardiac abnormalities. An example is given in Figure 2. The volume was then rendered using the EasyVision workstation (Philips Medical Systems, Best, The Netherlands) (Fig. 3).
It was possible to reconstruct both systolic and diastolic images of intracardiac anatomy or large vessels. Figure 4 compares these with reconstruction from the gadolinium enhanced magnetic resonance angiography in the same study.

Figure 2. Axial slices showing univentricular connection to a dominant left ventricle with absence of the left atrioventricular connection, hypoplasia of the left-sided rudimentary and incomplete right ventricle, and discordant ventriculo-arterial connections. (a) shows the coronary sinus entering the right atrium. (b) shows how the right atrium is connected to the dominant left ventricle, with no connection between the left atrium and the left-sided incomplete right ventricle. (c) shows the atrial septal defect in the oval fossa, while (d) shows the posterior pulmonary trunk and the left-sided anterior aorta.

Figure 3. Volume rendering of images acquired from the patient in Figure 2.
We have shown that it is possible to acquire high-resolution three-dimensional magnetic resonance volumes of the heart in a large population of patients of differing ages with different congenital cardiac malformations. This allows fast assessment of intra-cardiac structure and the anatomy of the large vessels in patients with congenital heart disease without complex planning. In addition, the improved contrast and signal homogeneity of these images allows simple thresholding segmentation to be used, significantly reducing post-processing time. This technique can also allow assessment of global and regional wall motion of the heart, as volume data is acquired in up to 10 phases of the cardiac cycle. All of this can be achieved within a reasonable scan time, compatible with a routine cardiac magnetic resonance study in these patients.

Figure 4. Volume rendering of the pulmonary trunk and its bifurcation as obtained (a) using diastolic images and three-dimensional steady state free precession, (b) with systolic three-dimensional imaging steady state free precession, and (c) with gadolinium enhancement.
Currently, two methods are used to assess three-dimensional cardiac anatomy. The multi-slice method has been shown to be an accurate way of assessing intra-cardiac structures.3 Gadolinium enhanced magnetic resonance angiography, on the other hand, is widely used in vascular imaging. Three-dimensional steady state free precession acquisition has advantages over both multi-slice acquisition and gadolinium enhanced magnetic resonance angiography. The major drawback of gadolinium enhancement, usually performed without cardiac synchronisation, is blurring of the images due to cardiac motion. This affects the ability of this technique to visualise intra-cardiac anatomy, and to a lesser extent the structure of the large vessels, such as the proximal pulmonary trunk and aorta. The multi-slice technique is cardiac gated, and thus blurring of images due to cardiac motion is reduced. As we have shown previously, this is an excellent method of both intra-cardiac and vascular visualisation.3 However, unlike the technique of gadolinium enhancement, which is acquired in a single breath-hold, multi-slicing requires multiple breath-holds, which leads to misregistration of the slices. In addition, volume rendering requires time-consuming manual segmentation of each slice. Three-dimensional steady state free precession is a fast technique for imaging, unlike the multi-slice method, and is cardiac gated, and therefore less susceptible to cardiac motion, unlike gadolinium enhancement. Importantly, the high “through plane” resolution, high image contrast, and excellent alignment of slices, make segmentation and rendering more straightforward. These factors make this technique the ideal one for visualisation of intracardiac structures, and allow visualisation of the large vessels without contrast. In fact, in some patients who were unwilling to undergo venous cannulation or receive contrast, we were successfully able to visualise large vessels only using the three-dimensional steady state free precession technique. If this trend continues in the future, it will also have the advantages of considerable savings in costs, because of the expense of contrast agents and elimination of the occasional side-effect from their use.
The other important advantage of this new technique is that it can be performed by the technologist, not requiring an experienced cardiologist to be present during the acquisition of images, even in cases with complicated anatomy. It is still important, nonetheless, that the technologist has experience in cardiac magnetic resonance to optimise the parameters used during scanning. The data can be analysed following the acquisition of images so as to reformat planes of interest, or volume render to obtain the information required for subsequent management of the patients. This also has the advantage that unsuspected finding or abnormalities will not be missed simply because imaging in a particular plane was not performed.
Current innovations in partially parallel imaging, and better use of the temporal information in the data, are leading to further reductions in scan time,6 and soon it will be possible to obtain volumes of similar quality to those illustrated in our figures in a single breath-hold.
Management of patients with congenital heart disease has the potential to be dramatically improved with widespread use of this new technique, allowing easier diagnosis and planning of treatment. As there is no need for expert planning when images are acquired using three dimensional steady state free precession, and most centres now have access to magnetic resonance imaging scanners, there is the potential for speedy dissemination of this technique.
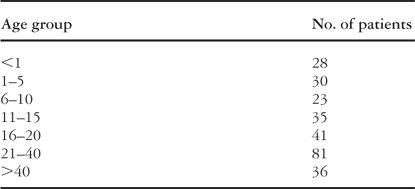
Table 1.

Reformatted image of the right ventricular outflow tract (a) without respiratory navigator and (b) with respiratory navigator.
Axial slices showing univentricular connection to a dominant left ventricle with absence of the left atrioventricular connection, hypoplasia of the left-sided rudimentary and incomplete right ventricle, and discordant ventriculo-arterial connections. (a) shows the coronary sinus entering the right atrium. (b) shows how the right atrium is connected to the dominant left ventricle, with no connection between the left atrium and the left-sided incomplete right ventricle. (c) shows the atrial septal defect in the oval fossa, while (d) shows the posterior pulmonary trunk and the left-sided anterior aorta.

Volume rendering of images acquired from the patient in Figure 2.

Volume rendering of the pulmonary trunk and its bifurcation as obtained (a) using diastolic images and three-dimensional steady state free precession, (b) with systolic three-dimensional imaging steady state free precession, and (c) with gadolinium enhancement.