Published online by Cambridge University Press: 22 April 2005
The lesion producing the typically fixed form of obstruction within the left ventricular outflow tract seemingly changes and evolves after birth. As we will discuss, patients exhibiting this characteristic exhibit some aspects of both acquired and congenital cardiac malformations. In these respects, canine subaortic stenosis is remarkably similar, especially as seen in the Newfoundland breed. Indeed, there is now a considerable literature devoted to subaortic stenosis in several mammalian species, including the cow and swine as well as the dog.1–11 As far as the dog is concerned, the Newfoundland is but one of a number of breeds found to harbour the fascinating lesion that obstructs the left ventricular outflow tract. The lesion has also been found in boxers, rottweilers, golden retrievers, German shepherds, German shorthaired pointers, and Bouviers de Flandres.6–11 In this review, we will discuss in detail this fixed type of subaortic stenosis as seen in the setting of an intact ventricular septum, addressing the underlying pathology, the evolving methodologies of investigation, and the results after surgical intervention. When germane, we will make comparisons with knowledge of subaortic stenosis as seen in the dog, particularly the Newfoundland. We have excluded from discussion, however, those patients with an atrioventricular septal defect in the setting of common atrioventricular junction.
The Newfoundland dog figures importantly in discussions about subaortic stenosis. The Newfoundland breed,12, 13 during the 19th century, became a status symbol for European society. Indeed, at one time this particular dog was the most popular canine import to Great Britain. The dogs themselves became instantly recognizable after Sir Edwin Landseer painted one of their numbers in 1838. The portrait was entitled “A Distinguished Member of the Humane Society” (Fig. 1). The dog in question was called Paul Pry, Landseer was paid £80 for his efforts, and the painting was eventually dedicated to the Royal Humane Society.14, 15

Figure 1. The painting of Paul Pry made by Landseer, and sold for £80.
It is the report of Norman C. Chevers, published in 1842, which is likely the earliest description of subaortic stenosis in man.16 More than 80 years later, Sir Arthur Keith, in his 1924 Schorstein Lecture on the fate of the bulbus cordis, provided a wonderful drawing of a specimen from a 20-year-old man with a constricting ring below the orifice of the aorta.17 Keith opined that a remnant of the incompletely absorbed bulbus cordis provided the origin of the subaortic ridge. Abbott, in her now famous “Atlas of Congenital Heart Disease”18 published in 1936, lists 12 of her 1,000 cases as having subaortic stenosis, her patients ranging in age at death from 2 years to 58 years, with a mean of 23 years. Despite this early attention, however, the disorder does not receive mention in either of the first editions of the textbooks of Paul Wood and Helen Taussig, nor can any discussion be found in the earliest edition of Nadas' Pediatric Cardiology.
Regarding the dog, Detweiler and Patterson1 have provided an important narrative of the overall history of canine cardiology. According to them, by 1913, descriptions had been provided of the chief clinical signs of valvar and myocardial disease in dogs and, by 1929, the pathology of some forms of cardiovascular disease had been extensively reviewed. In terms of congenital malformations, when Patterson catalogued the pertinent literature in 1971, he found that, over the period from 1800 to 1970, 255 dogs had been reported to have 276 separate cardiovascular defects, of 23 different types.6 Subsequently, both hypertrophic and dilated variants of cardiomyopathy have been well described, with the dilated variant being frequently seen in the Newfoundland.19–21 Subaortic stenosis has also been noted complicating interruption of the aortic arch.22 It was Patterson's colleague Detweiler, also working at the University of Pennsylvania, to whom Patterson credits for promoting the idea that the study of cardiovascular diseases occurring naturally in the dog might provide insight into the causes and pathogenesis of similar conditions in man.1, 2 As we now survey the literature devoted to congenital cardiac disease as seen in the dog, we see the validity of this concept, since the unfolding cascade of knowledge reflects in many respects the contemporary practice of paediatric cardiology in man. For example, the information on the interpretation of low-intensity murmurs in dogs predisposed to subaortic stenosis,23 is reminiscent of many of the clinical publications in paediatric cardiology of the 1940s and 1950s. With the ongoing discoveries relative to the canine genome, we anticipate major advances in the near future. With regard to the specifics of canine subaortic stenosis, however, the situation is somewhat less precise, albeit that the contemporary literature is extensive, documenting the pathology and genetics in multiple breeds, as well as providing information regarding the clinical course.1, 2, 4–11
The left ventricular outflow tract extends from the free edge of the aortic leaflet of the mitral leaflet to the semilunar attachments of the aortic valvar leaflets. It is bounded anteriorly by the muscular ventricular septum, and posteriorly by the aortic, or anterior, leaflet of the mitral valve. The aortic leaflet of the mitral valve is continuous at its superior extent with the non-coronary and right coronary leaflets of the aortic valve, this extensive area being variously described as the region of fibrous mitral-to-aortic valvar continuity, the intervalvar septum, or the area of mitral-aortic separation. It was Rosenquist et al., some years ago now,24 who documented the spectrum of normal separation between the valvar leaflets as extending from 0 to 10 millimetres. This separation is often exaggerated in patients with the fixed form of subaortic stenosis.25 A rare formation of a congenital subaortic aneurysm can also extend from this fibrous area.26 Similar abnormal subaortic infundibular morphology has also been documented in the dog as contributing to subaortic obstruction.27
Most patients with the fixed form of subaortic stenosis have a left-sided heart, normal atrial arrangement, concordant atrioventricular and ventriculoarterial connections, a normal subpulmonary infundibulum, normal spatial relationships between the aortic roots, and a left aortic arch. As we discuss later in this review, nonetheless, some patients with the tunnel form of subaortic stenosis may exhibit important fibrous discontinuity between the mitral and aortic valves, albeit whilst maintaining normal, or nearly normal, spatial relationships between the arterial roots.
Many terminologies have been used for description of the so-called fixed lesion that obstructs the left ventricular outflow tract in the setting of an intact ventricular septum. In this regard, when discussing the best terminology for hearts characterized by a common atrioventricular junction, Becker and Anderson28 posed the question “What's in a name”? The same question can be posed relative to all these terms used to describe the relatively simple arrangement of the lesion producing fixed subaortic obstruction (Table 1).
Table 1. Terminologies used for so-called “fixed” subaortic stenosis.

Somerville has emphasized, in a number of publications,29–31 that the lesion producing the stenosis is rarely, if ever, discrete. Nor is it “membranous”, and rarely is it thin. The offending substrate is usually a fibromuscular ring, or shelf, with dimension of 1 to 2 millimetres or more, which forms a crescentic and nearly circumferential diaphragm at a variable distance beneath the aortic valve (Fig. 2). The suggestion that the obstruction is thin, discrete, or membranous, probably stems from its appearance on angiography. As Somerville31 points out, this is not what is observed at surgery, or at necropsy. Admittedly, as seen at necropsy, certainly in those individuals requiring operation, or in those who have died because of severe and longstanding subaortic obstruction, the lesion can have evolved considerably from its native form. The avascular shelf is firm and non-distensible. It typically courses from the left ventricular septal surface to the ventricular surface of the aortic leaflet of the mitral valve (Fig. 3). Rarely, two separate ridges have been described, albeit an echocardiographic finding.32

Figure 2. Gross appearance of fixed short-segment subaortic stenosis. (a) The offending subaortic tissue (*) is viewed from the left ventricle with the aortic valve intact. The subaortic stenosis forms a nearly complete ring. (b) The appearance of the subaortic obstructing tissue (*) with the left ventricle (LV) opened through the thickened aortic valve (AO) leaflets. AMVL: anterior leaflet of mitral valve; MV: mitral valve; TAV: thickened aortic valve.

Figure 3. Diffuse, long-segment subaortic stenosis with fibromuscular obstruction. (a) There is extensive deposition of endocardial sclerotic tissue (black asterisks) in the subaortic area, with a muscular septal bulge (white asterisks) contributing to the obstruction. Note the involvement of the aortic valve leaflet (V). (b) The right axial oblique left ventriculogram of the patient whose heart is shown in Figure 3a. The angiography suggests a subaortic chamber (*), with narrowing (white arrows) beneath it. The angiogram does not indicate the extent of endocardial thickening. (c) Aortic–mitral separation in a dog with long-segment subaortic stenosis. AO: aorta; OT: left ventricular outflow tract; arrows: denoting long-segment subaortic stenosis; LV: left ventricle; MV: mitral valve. Figure 3c modified with permission from Ref. 56.
In an ultrastructural study, Ferrans et al.33 identified five specific layers of tissue within the shelf. These were a surface monolayer of endothelial cells, a sub-endothelial layer rich in acid mucopolysaccharides and basement-membrane-like material, a fibroelastic layer containing collagen and small elastic fibres, a layer of smooth muscle cells with thickened basement membrane, and a central fibrous layer containing large amounts of collagen and small amounts of elastic fibres. Their observations were extended by Allen et al.,34 who studied histologically the segments removed after septal myectomy in patients known not to have hypertrophic cardiomyopathy, yet still found evidence of myocardial disarray.
Marked separation of the attachments of the leaflets of the aortic and mitral valves is a feature of some patients with so-called short segment fixed subaortic stenosis, but such separation is typically more conspicuous in those having diffuse, or tunnel-like, stenosis.25 A fibromuscular collar can also contribute to such elongated types of fixed stenosis (Fig. 3). Interestingly, severe subaortic obstruction has been described in patients with so-called anatomically corrected malposition. This condition is characterized by concordant ventriculoarterial connections, but with marked separation of the aortic and mitral valves due to persistence of a subaortic infundibulum, usually with the aorta in anterior and left-sided position.35–38
Severe subaortic stensosis can also, of course, be found in combination with deficient ventricular septation.35–40 In this setting, other structures may also contribute to narrowing of the left ventricular outflow tract, either singly or in combination, including accessory tissue tags derived from the mitral valvar leaflets, malattachment or muscularization of the mitral valve, abnormal insertion of its papillary muscles, posterior deviation of the muscular outlet septum, or some combination of the above.39–50 While of obvious interest, these are beyond our present scope, since we are concerned with fixed stenosis in the setting of an intact ventricular septum.
In those patients with intact ventricular septal structures, there have been several classifications offered for the specific lesions producing stenosis, with most differentiating between dynamic or muscular forms as opposed to the fixed forms. In many respects, the differences reflected the imaging modality used to provide the classification.51–53 In the relatively simple systems, some have advocated distinguishing discrete from diffuse or tunnel forms, while others have recognized discrete, fibromuscular, and tunnel variants. Still other systems are more complex. Deutsch et al.54 suggested that the discrete lesions could take 4 patterns, a thin shelf-like type of stenosis localized at the very cranial portion of the left ventricular outflow tract at the level of the attachment of the aortic valve, a thicker fibrotic annular stenosis, from 2 to 3 millimetres thick, producing a straight linear translucency approximately 2 centimetres below the aortic valve, a fibromuscular type with an irregular appearance due to disseminated small polypoid masses or ridges bulging into the left ventricular outflow tract just below the attachments of the leaflets of the aortic valve, and an irregular but fixed tunnel-like formation involving the entire left ventricular outflow tract. Kelly et al.,55 using angiography, found that two forms were enough for descriptive purposes, specifically a thin and discrete shelf as opposed to fibromuscular stenosis associated with muscular hypertrophy and narrowing of the outflow tract. Using echocardiographic imaging, but including patients with a ventricular septal defect, Choi and Sullivan53 reverted to four types. Their choices for description were short-segment, long-segment, posterior displacement of the muscular outlet septum, and redundant tissue arising from the membranous septum (Figs 3 and 4).

Figure 4. Histopathology of offending subaortic substrate in man. (a) Histological whole-mount section of the subaortic interventricular septum in the longitudinal plane showing severe subaortic endocardial fibroelastosis (arrows). (Movat pentachrome stain). (b) Photomicrograph of subaortic region of the interventricular septum showing severe (2–3 mm in thickness) endocardial fibroelastosis that shows abundant dense collagen (green stain) but relatively little elastic fibre formation (black). The underlying myocardium shows minimal change with a band of subendo-cardial fibrosis and normal myocardium within the interventricular septum (elastic trichrome stain).
Descriptions for the gross appearances of the subaortic shelf as seen in a number of canine breeds, including the Newfoundland and Boxer, are remarkably similar to those provided for man.7–10 Although not specifically mentioned, separation between the hinges of the leaflets of the aortic and mitral valves may also be significant in the Newfoundland dog, as shown in Figure 6a of Patterson's publication of 198456 (Fig. 3c). King et al.57 found an incomplete subaortic ring in several domestic animals, suggesting this to be a newly described congenital anomaly. Patterson and colleagues, nonetheless, had earlier stated in several publications7–10, 56 that the ring may be incomplete, so this finding may not be truly “newly described”. Ultrastructural studies of the fibrous subaortic shelf from Newfoundland dogs58 have revealed significant differences from the findings in the human form.33 In the dog, the shelf is characterized by the presence of large, uni- and multinucleated, rounded connective tissue cells that resemble chondrocytes. Connective tissue adjacent to these cells is rich in acid mucopolysaccharides, possesses small but cross-banded collagen fibrils, and small, poorly developed elastic fibres. The chondrocytic-like cells contain numerous cisterns of rough surfaced endoplasmic reticulum and prominent Golgi complexes, and they are surrounded by thick, concentrically arranged layers of basement membrane.

Figure 5. Relatively ‘discrete’ examples of fixed, short-segment subaortic stenosis as imaged from left ventriculography. (a) Retrograde left long axial oblique left ventriculogram shows a linear obstructing ‘ring’ (arrows) beneath the aortic valve. (b) A different patient whose fixed subaortic tissue (arrows) is even closer to the aortic valve.
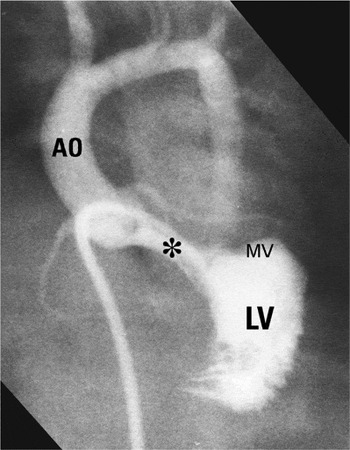
Figure 6. Abnormal cardiac “scaffold” with minimal systolic pressure gradient between left ventricle and aorta. Note the elongated subaortic outflow tract (*) with aortic-mitral separation. This patient does not have an atrioventricular septal defect.
Involvement of the valvar leaflets in the lesion producing fixed stenosis, however, has been well documented in both dog and man.6–11, 56–59 In some of these cases, the extensions deform the leaflets of the aortic valve, likely being responsible for the accompanying aortic valvar incompetence.59
There is little, if any, mention of involvement of the coronary arteries in humans, either as a primary or secondary process. This is very different in dogs, where subaortic stenosis is often accompanied by extensive alterations of the intramural coronary arteries within the left ventricle, albeit not in the intramural coronary arteries of the right ventricle, nor the extramural arteries.60 The coronary arterial lesions do not seem age-related, being more severe in many dogs under two years of age than in older dogs. The lesions themelves consist of fibrous intimal proliferation accompanied by fibrous replacement of medial smooth muscle. The internal elastic lamina is often duplicated, occasionally demonstrating hyperelastosis. In other arteries, the internal elastic lamina is fragmented, while yet in others it has disappeared. Thus, it should not be surprising that subendocardial myocardial necrosis may be conspicuous in dogs with subaortic stenosis, particularly since there is reversed flow throughout systole in the circumflex coronary artery.61, 62
As of 1958, the classic textbook of Keith, Rowe and Vlad considered isolated subaortic stenosis a rare anomaly in man, stating that, to 1949, only 45 cases had been cited in the literature.63 Indeed, they noted that, of 7,650 autopsies performed at Toronto's Hospital for Sick Children during the preceding 30 years, only one case had been seen. Wood also considered subaortic stenosis to be rare, although he commented that the majority with this condition die young.64 It is now thought, however, that subaortic stenosis accounts for up to three-tenths of all cases of obstructed left ventricular outflow tracts seen in children.65, 66 A reliable figure for incidence is provided by the recent Maltese study carried out using cross-sectional echocardiography. This investigation revealed an incidence for primary subaortic stenosis of 0.25 for each 1,000 live births.67 Questions remain concerning the results of earlier studies predating the era of cross-sectional echocardiography.68, 69 Because these studies relied on auscultatory findings, and with these being similar in patients with subaortic stenosis to those found in patients with small ventricular septal defects, it is unclear whether the studies based on clinical findings would underestimate or overestimate the prevalence, but we would expect them to produce an overestimate.
Discussion continues as to whether the fixed forms of subaortic stenosis are congenital or acquired cardiac anomalies. Much evidence lends credence to the lesion not only being congenital, but also having a genetic background.65–93 Thus, the finding that coarctation of the aorta, bifoliate aortic valves, mitral valvar anomalies, and/or a ventricular septal defect occur in more than half the patients having fixed subaortic stenosis points to the implication of congenital factors in the pathogenesis.30, 65, 66, 77–93 Other supportive findings include the fact that some cases, admittedly uncommonly, are diagnosed in the neonatal period,86–89 there is well-recognised recurrence in siblings,84 and the fact that some patients with fixed subaortic stenosis have been born to non-affected but consanguineous parents. On the basis of these observations, it has been suggested that fixed subaortic stenosis could be the result of an autosomal recessive mutation90, 91 (Table 2).
Table 2. Evidence supporting a congenital and genetic basis.

From the stance of the dog, Patterson, his colleagues, and others, have discussed extensively the epidemiologic and genetic aspects of cardiovascular development,4–7, 10, 94, 95 while Mulvihill and Priester97 have reiterated the similarities between canine and human congenital cardiac disease. Taken overall, the frequency of congenital cardiac disease in the dog as estimated from these studies is roughly 5 per 1,000. This is similar to the rate amongst human infants reported by Mitchell et al.98 It is likely, nonetheless, that this study somewhat underestimates the frequency, excluding as it does the milder malformations, a problem also found in other earlier studies of human populations. In the dog, however, cardiovascular malformations are significantly more common in purebred dogs than in mongrels. Thus, the rates of congenital heart disease have been estimated at 2.6 per thousand in mongrels, to 8.9 per thousand in those that were purebred.5, 6, 95 Furthermore, different breeds differed significantly in their risks for specific defects.1–6 In the Newfoundland, the prevalence for all kinds of congenital cardiac disease is 7.81 per 1,000, falling to 6.35 per thousand when adjusted for age,1–6 and significantly, the commonest lesion seen in the Newfoundland is subaortic stenosis.8–10, 94, 95 The commonest canine congenital cardiac anomaly in Sweden is also valvar or subaortic stenosis.96 All these studies suggest that the common forms of congenital heart disease in dogs have an important genetic component. Indeed, it seems that five of the most common defects are inherited as specific traits. While the type of malformation is specific, however, the severity of the malformation is variable, from subclinical variants to severe or lethal forms. Patterns of inheritance are consistent with a polygenic threshold model, in which multiple genes act additively, and with the incidence and degree of severity increasing with the dose of genes predisposing to the defect.
Considering subaortic stenosis specifically, studies in the Newfoundland dog8–10, 56, 94, 95 show this to be a specific inherited trait, with a complex polygenic or autosomal dominant mode of transmission. The lesion has also been recognized as an inherited lesion in British boxers for nearly 40 years. Indeed, in 1991 nearly half of all dogs referred to the Royal School of Veterinary Study in Edinburgh with subaortic stenosis were boxers.99 Because of this, a scheme to control breeding has been in operation in the United Kingdom since 1990.99
As discussed above, there are many reasons why the fixed forms of subaortic stenosis could be considered a congenital abnormality. We should also note, however, that as yet the lesion has not been documented in fetal life. This, coupled with the fact that most patients are detected after infancy, leads others to consider fixed subaortic stenosis an acquired disorder.70–75 Indeed, re-examination of the classic paper of Pyle, Patterson and Chacko8 that addressed the situation in the dog, coupled with other obervations in the Newfoundland, particularly concerning the timing of the development of the subaortic shelf, show that this pathology is not truly a congenital abnormality, but rather one that develops postnatally.9, 10, 60, 94, 95 Could it be, therefore, that the etiology of the fixed forms of subaortic stenosis has both congenital and acquired aspects, beginning with an abnormal scaffold that provides the basis for formation of the eventual stenotic lesion? In this regard, we find the hypothesis proposed by Cape et al.100 to be compelling. They identified four components as contributing to the development of subaortic stenosis in the human. The first predisposing factor is the presence of morphologic abnormalities within the outflow tract. These lead to the second factor, an elevation of septal shear stress. If this is coupled with the third potential factor, namely a genetic predisposition, and then excacerbated by the fourth factor, cellular proliferation in response to septal shear stress, the result is the fixed and stenotic lesion. As regards the potentially predisposing morphologic abnormalities, these can include, either singly or together, some degree of separation between the leaflets of the aortic and mitral valves, perhaps representing persistence of the left-sided ventriculoinfundibular fold, a small aortic outflow tract and aortic root, left ventricular bands, mitral valvar pathology, and a steeper aortoseptal angle.25, 101–108 Thilenius et al.,101 and El Habbal and Suliman,102 found that the aortic root was small in one-quarter of their patients with fixed subaortic stenosis. Such elongation and narrowing of the left ventricular outflow tract, coupled with a steeper aortoseptal angle, are known to produce important changes in septal shear stress.109–114 The common denominator for all these abnormalities is that they produce chronic turbulent flow in the left ventricular outflow tract, setting the scene for an abnormal fibrous response at the endothelial interface, with an increase in turnover of endothelial cells.109, 112, 113
Significantly, a steepened aortoseptal angle has also been shown to be predictive of the development of subaortic stenosis in patients with a ventricular septal defect.106–108 This should not be surprising, considering the frequency of a subaortic ridge or deformity in patients with either a perimembranous ventricular septal defect, coarctation of the aorta, or both.115 Further evidence supporting the role of turbulent flow of blood in the etiology and genesis of fixed subaortic stenosis is the observation of both subpulmonary and subaortic ridges in patients with the doubly committed subarterial ventricular septal defect.116 One reason why the typical findings of the fixed forms of subaortic stenosis are not commonly found in the newborn could be that fetal endocardial tissue is less likely to demonstrate a rich fibrous reaction or proliferation to shear stress than postnatal endocardium.30 As yet, however, this suggestion is no more than a supposition, albeit an attractive one. It remains our own opinion, nonetheless, that the development of the fixed forms of subaortic stenosis is likely influenced by both congenital and acquired factors (Table 3).
Table 3. Morphological features contributing to an abnormal cardiac “scaffold”.

Many of these observations are equally germane to those patients with an associated ventricular septal defect. As emphasized, thus far we have excluded this latter group from discussion. This is because the presence of a ventricular septal defect, either perimembranous or subarterial outlet, may in itself set the stage for turbulent flow in the left ventricular outflow tract.115, 116 This may account for the prevalence of a subaortic ridge in those patients with a perimembranous ventricular septal defect and anomalous muscle bundles of the right ventricle.81 For the reasons pointed out by Anderson in a letter to the editor,117 we have excluded from our present discussion those patients with fixed subaortic stenosis in the setting of an atrioventricular septal defect with common atrioventricular junction.
The marked progression of the fixed forms of subaortic stenosis is well recognised.30, 53, 65, 66, 70–76, 100, 109–114, 118–123 Although studying only a small group of patients, Mody and Mody118 provided a good review of the pertinent literature. Evidence demonstrating progression was provided by Newfeld et al.77 using cardiac catheterization, and also by Wright et al.65 The latter group found that the gradient across the left ventricular outflow tract increased in 25 of 26 patients catheterized more than once before surgery. Shem-Tov et al.119 used echocardiography to address the clinical presentation and natural history of the milder variant. They followed 21 patients with a peak systolic gradient across the left ventricular outflow tract of less than 50 millimetres of mercury for from 1 to 17 years. Subacute bacterial endocarditis occurred in 3 patients, while 10 had aortic insufficiency. In 3 of these 10 patients, aortic insufficiency was found only at the second catheterization. Of the eight patients who were re-catheterized, one-third of the entire group, seven showed an increase in gradient. We also used serial catheterisation120 to establish the progressive nature of the stenosis, finding similar progression in patients both with an intact ventricular septum and in those with a ventricular septal defect. Frommelt et al.121 used serial echocardiographic studies to demonstrate significant progression of the gradient across the left ventricular outflow tract, as did Choi and Sullivan.53 Rohlicek et al.,123 nonetheless, reported that many children with mild subaortic stenosis exhibit little progression of obstruction or aortic regurgitation, and need not undergo immediate surgery. Others, with more severe subaortic stenosis, however, may progress precipitously, and will benefit from early resection despite risks of surgical morbidity and recurrence. The stenosis is less likely to progress in the adult, or if progression does occur, it does so more slowly.124 In this regard, Oliver et al.124 showed a significant relationship between obstruction within the left ventricular outflow tract and the age of the patient. Taken overall, the majority of studies using either serial cardiac catheter investigations or cross-sectional echocardiography with Doppler interrogation show that the fixed forms of subaortic stenosis progress not only in hemodynamic severity, but also change their form.
Thus, the recent advances in imaging have permitted considerably closer scrutiny of the evolution of the disorder over time in regard to its severity, its form, and the appearance and progression of aortic regurgitation (Figs 3, 5–7). Both transthoracic and transesophageal echocardiographic imaging (Figs 8–10) has facilitated the appreciation of those morphologies.51, 53, 74–76, 121, 125, 126 Without resorting to postoperative cardiac catheterization, intra-operative and postoperative studies permit ongoing assessment of the adequacy of relief of the obstruction, and demonstrate any inadvertant perforation of the ventricular septum, reveal any mitral annular aneurysm, or exclude iatrogenic mitral regurgitation. If identified, the latter complication can be dealt with promptly.127, 128 Cross-sectional imaging has now extended also to canine cardiology.129, 130 In the dog, as in the human, the progression in severity varies considerably. Some suggest that progression in the dog is more rapid when the stenosis is subaortic than for aortic valvar stenosis. In man, on occasion, the progression can be very rapid, indeed dramatic, with a fatal outcome.131, 132 In a patient we reported some years ago,131 the angiocardiographic appearance of the left ventricular outflow tract was initially considered to be normal, albeit that we had failed to note the elongation produced by persistence of the left-sided ventriculo-indundibular fold producing discontinuity between the leaflets of the aortic and mitral valves, and thus providing the substrate for turbulent flow of blood (Fig. 11).

Figure 7. Examples of long-segment subaortic stenosis. (a) This patient has important aortic–mitral (MV) separation (white arrows), with a septal bulge (white asterisk) contributing to the tunnel (black asterisk) subaortic stenosis. (b) A different patient with recurrent and unrelieved severe tunnel (large black asterisk) subaortic stenosis following earlier transaortic approach (small black asterisks note site of aortotomy). A septal bulge contributes to the obstruction (white arrows).

Figure 8. Echocardiograms showing short-segment, fixed subaortic stenosis. (a) Five-chamber view shows what is considered a so-called “discrete membrane” (arrows) below the aortic valve. (b) Colour Doppler image in parasternal long axis view shows flow acceleration (asterisk) starting at the level of the offending obstructive substrate. (c) Continuous wave Doppler tracing shows increased velocity above the level of the short-segment fixed obstruction. (d) Parasternal long axis view in diastole shows a regurgitant jet (arrows) through the aortic valve. AO: aorta; LA: left atrium; LV: left ventricle.

Figure 9. Echocardiograms showing tunnel subaortic stenosis. (a) Parasternal long axis view shows long segment narrowing (asterisks). There is discontinuity between the mitral and aortic valve leaflets. The arrows indicate hinge points of the mitral and aortic valve leaflets. (b) Colour Doppler image in a corresponding plane shows flow acceleration through the tunnel obstruction (asterisks). Refer to Figure 8 for abbreviations.

Figure 10. Echocardiogram in parasternal long axis view shows subaortic stenosis secondary to accessory tissue (arrow) arising from the mitral valve. Refer to Figure 8 for abbreviations.

Figure 11. Rapid development of subaortic stenosis following repair of coarctation of the aorta. (a) Before repair of the coarctation when the patient was 8 weeks of age, the left ventricular outflow tract was elongated with aortic–mitral separation (white asterisks), consistent with an abnormal cardiac “scaffold”. No pressure gradient was recorded between the apex of the left ventricle and ascending aorta. (b) Following repair of the coarctation with a subclavian flap aortoplasty (short white arrows), the patient developed very severe tunnel subaortic stenosis (black arrow). This catheter study was performed when the patient was 6 months of age. The level of the aortic valve is noted with a single long white arrow. The pressure in the left ventricle distal to the fibromuscular obstruction was 252/10 mmHg and in the ascending aorta, 88/52 mmHg. Figures 11a and b modified from Ref. 131.
In the face of such variability in natural history, the question is whether we can recognize the specific forms of fixed and short segment stenosis that are likely to progress? Attempting to answer this question, Bezold et al.133 developed and validated a predictive echocardiographic model. Multivariate analysis using the model identified 3 independent predictors of progressive disease, specifically the indexed distance from the leaflets of the aortic valve to the subaortic shelf, the involvement of the aortic leaflets of the mitral valve, and the initial gradient as measured at Doppler interrogation. When the equation derived from the model was tested in patients, a predicted value of less than 0.58 yielded sensitivity and specificity both of 100% for distinguishing progressive from non-progressive variants of stenosis. Patients with intermediate levels of progression could not be distinguished from those showing unequivocal progression, but were clearly separable from those patients who did not progress. In the 6 years that have elapsed since this important publication, however, we can find no subsequent publication validating their regression equation in a prospective study using a large cohort of patients.
The demographics and natural clinical history of subaortic stenosis have been extensively evaluated by retrospective analysisin the dog.134 Nearly 200 animals were confirmed as having subaortic stenosis, and 96 of these were untreated and available for follow-up evaluation, with systolic pressure gradients suitable for assessment of severity available from 58 dogs. All the dogs were included in a demographic analysis. Breeds found to be at increased relative risk included the Newfoundland, Rottweiler, Boxer, and Golden Retriever. Dogs with milder gradients, and those that developed infective endocarditis or left heart failure, were diagnosed at older ages than those with moderate or severe gradients. Of the dogs that were not treated, one-third had signs of illness, varying from fatigue to syncope. Infective endocarditis or left heart failure developed on just over one-tenth, usually later in life, and in the setting of milder stenosis. In addition, one-fifth of the cohort died suddenly, with sudden death occurring mainly in the first 3 years of life, primarily but not exclusively, in dogs with severe obstructions. The dogs with mild obstruction lived longer than other groups, and tended to remain asymptomatic. Thus, in dogs, the prognosis for long-term survival is favorable for untreated mild or moderate fixed stenosis, but is poor when the stenosis is severe. Progression has also been documented in the boxer,135 with some of these dogs developing aortic and even mitral regurgitation.
If we compare the findings, therefore, there is considerable evidence in man to support the notion that short-segment stenosis evolves to become a longer and more diffuse form of fixed subaortic stenosis. The most exquisite information showing the morphologic appearances of such progression, nonetheless, comes not from studies in man, but from the studies of Patterson in the Newfoundland dog.7–9, 56 These studies showed that the mildest form of stenosis was no more than a number of whitish, slightly raised, nodules on the endocardial surface of the ventricular septum immediately below the aortic valve. These progressed to produce a narrow ridge of whitish, thickened endocardium that extended partially around the left ventricular outflow tract. Such ridges had a variable location, but in most cases they originated at the base of the aortic leaflet of the mitral valve and extended transversely across the ventricular septum beneath the left coronary aortic sinus. The most severe lesions took the form of a fibrous band, ridge, or collar that encircled the left ventricular outflow tract just below the aortic valve. The shelf was often raised above the endocardial surface, and now extended across the ventricular septum beyond the left coronary aortic sinus to reach the base of the aortic leaflet of the mitral valve. When the subaortic lesion was most severe, the ventricular surfaces of the aortic valvar leaflets were also thickened. These important studies also showed that lesion could not be detected in any puppy less than three weeks of age when the left ventricular outflow tract was studied with a dissecting microscope.
Aortic regurgitation is known to develop in the clinical course of subaortic stenosis in both man and dog, some suggesting the complication to involve half or more of afflicted cases.65, 74–76, 93, 119–123 The reported incidence, at least in man, varies widely depending on the era of the publication and the modality of imaging used for its recognition. The etiology is probably multifactorial. Involvement of the base of the aortic valvar leaflets by the fibroelastic shelf unequivocally restricts their motion, while the leaflets are further distorted by the turbulent flow of blood through the narrowed subaortic outflow tract. Further changes can be produced secondary to infective endocarditis, should this occur.59, 74–76 Infective endocarditis is known to be a particularly common complicating factor in the Newfoundland and boxer.58, 134, 135 Further contributing factors in man have been shown to be bi-or quadrifoliate formation of the valve.136 It is also suggested that the distance between the obstructing shelf and the valvar leaflets influences the development of aortic regurgitation.137 The reported incidence of aortic regurgitation in patients prior to surgery, however, varies widely, depending on the age of the patient at the time of the assessment, the severity of the obstruction, and methodology of surveillance.75, 121, 122, 138, 139 In most of the larger series, the incidence of aortic regurgitation was about the same postoperatively when compared to the preoperative state. There has also been discussion as to how the type of surgery effects the postoperative incidence and severity of aortic regurgitation. Van Son et al.140 reported that the risk of late aortic regurgitation was much lower when myectomy was combined with removal of the shelf, than when the shelf was taken out in isolation, or in combination with myotomy rather than myectomy. As yet, the optimal timing of intervention to reduce the incidence and severity of aortic regurgitation has not been established.139–144 Brauner et al.145 have provided data suggesting the benefit of early surgical intervention, intervening before that gradient exceeds 40 millimetres of mercury. We reached somewhat different conclusions,146 finding that early intervention, while reducing the rate of recurrence of subaortic stenosis, did not reduce the frequency of acquired damage to the aortic valve. We should also remember that endocarditis is a further hazard and, if occurring, will promote worsening of any aortic regurgitation (see below).
Many have cautioned about the risk of infective endocarditis in patients with subaortic stenosis, both before and after surgery.65, 95, 119, 122 Any thickening of the aortic valvar leaflets likely increases the risk of endocarditis. The risk is clearly less today than in earlier eras, being reduced by careful surveillance of these patients, particularly with the use of transesophageal echocardiography, and the advent of appropriate antibiotic therapy. In the group of patients reported by Wright et al.,65 over half had mild aortic regurgitation prior to surgery at a median age of 12 years. Infective endocarditis occurred in one-eighth of the group, with a frequency of 14.3 cases per 1,000 patient-years. As already emphasized on several occasions, infective endocarditis is frequently observed in the Newfoundland with subaortic stenosis, and certainly contributes to worsening of the regurgitation.147
We have already discussed how involvement of the mitral valve can contribute to the etiology of subaortic stenosis, and we have listed earlier in this review those anomalies which may produce obstruction ot the left ventricular outflow tract. Our understanding of these conditions has evolved from the autopsy table to recognition using cross-sectional echocardiography. Cohen et al.42 examined the anatomy of the mitral valve echocardiographically, assessing its relationship to the other components of the left ventricular outflow tract in a series of 73 consecutive patients referred to their institution from 1994 to 2000 for surgical correction of discrete subaortic stenosis. In all patients for whom it was considered advisable, the mitral valvar anomaly was corrected surgically, together with resection of the fibromuscular subaortic stenosis. One or more abnormalities involving the mitral valve were found in almost half the patients. These involved insertion of a papillary muscle into the aortic leaflet or the ventricular wall across the outflow tract, muscularization of the aortic leaflet, anomalous insertion of the tendinous cords into the ventricular wall, or protrusion of accessory valvar tissue tags. Surgical correction was feasible in all cases with anomalous mitral valvar anatomy. They concluded that the incidence of mitral valvar anomalies is probably underestimated in the setting of subaortic stenosis. The mitral valve may also be intrinsically abnormal. Obstructive anomalies of the mitral valve, including the parachute deformity and the supravalvar stenosing ring, are well-known components of the Shone complex,148 while an isolated cleft can result in regurgitation.45, 46 The pathology of the papillary muscles in patients with discrete subaortic stenosis and mitral regurgitation, however, has received only modest attention, with Allwork and Restivo149 demonstrating that myocytolysis, vasculitis, and fibrosis can contribute to mitral valvar dysfunction, especially in the young patient. In similar fashion, little attention has been given to the position of the subaortic shelf relative to mitral valvar function. In this respect, Paul et al.150 found that the distance between shelf and valvar leaflets was significantly greater in the group of patients with mitral regurgitation. They speculated that tethering of the mitral valvar leaflet prevents normal coaptation. Lending support to this suggestion is the observation that mitral regurgitation usually resolves after surgical intervention. After such resection of the subaortic lesion, it is known for mitral annular aneurysms to develop, albeit rarely.128 The aneurysm was attributed to disruption of the valvar continity at the initial operation. We have not seen this complication in the follow-up of our patients undergoing surgery in Toronto, although we did encounter the problems after relief of severe subaortic stenosis following earlier repair of an atrioventricular septal defect.151
There is considerable literature documenting mitral valvar dysplasia and regurgitation in the dog,152–155 albeit that congenital mitral stenosis is less common.
Discussions continue regarding the initial indications for surgery, the timing of surgery to prevent recurrence, the timing to prevent and reduce the incidence of aortic regurgitation, and the type of intervention. The latter is now the more important, since intervention has begun to evolve from exclusively surgical156 to catheter-based therapies,157, 158 although the use of balloon angioplasty remains controversial. Surgical intervention has a long history, beginning with the experience of Brock using transventricular dilation.159 It was Spencer et al.,160 who first reported in 1960, treatment with the use of cardiopulmonary bypass. The type of surgical intervention has continued to change over the years, in large part due to the issues of recurrence, and also with the aim of preventing ongoing damage to the aortic valve. It is difficult to define the precise incidence of recurrence due to the changing surgical technique, this problem being excacerbated by the varying methodologies used for postoperative surveillance. While it may be easy to relate recurrence to a given surgical technique, this ignores the fact that the intrinsic cardiac scaffold can set the stage for unequivoical recurrence unless radical reconstruction of the outflow tract can be achieved. This is particularly true for those patients with the diffuse, long-segment, tunnel form of stenosis.156, 161–163
Initial therapy for the short-segment type of stenosis took the form of excision of the obstructive fibrous shelf. This was later followed by removal of the shelf combined with myotomy, and then further evolved to myomectomy rather than simple myotomy.74, 83, 122, 138–144, 156, 163–169 Even today, discussion continues on the merits and necessity of the more aggressive forms of surgical intervention.162, 167, 168 In those patients with a short and fixed stenosis, as well as those with a conspicuous septal bulge, myomectomy is an important adjunct to removal of the fibrous shelf. Contemporary surgical mortality for removal of the shelf combined with myomectomy is low, less than 1 to 2 percent, with the complications of surgical heart block or ventricular septal perforation also usually less than 1 to 2 percent.156 Mobilization of the left and right fibrous trigones at the ends of the area of aortic-to-mitral fibrous continuity, as proposed and performed by Yacoub et al.,170 also carries similarly low surgical mortality and morbidity. We commented earlier about the strategy of early surgical intervention to prevent recurrence, a strategy advocated by Brauner et al.,145 emphasizing that our own observations lent no credence to this approach.146 Removal of the shelf combined with myotomy, however, will not suffice for the tunnel form of subaortic stenosis. For those having the short-segment form of stenosis, it is more likely the type of operation and cardiac scaffold, rather than the specific timing and gradient, that prevents recurrence. At the Toronto Hospital for Sick Children and the Toronto Hospital, we have now performed surgery on 202 patients with these forms of stenosis, using a variety of surgical techniques over the past 30 years. The median age at operation was 7.09 years. There were 2 early, and 12 late deaths. Our experience showed that recurrence is usually observed within a few months to a few years after the initial surgical procedure. In a few patients, however, recurrence approaching three decades has been recorded.171, 172 Fixed subaortic stenosis has also been observed to develop following spontaneous closure of a ventricular septal defect, or following its surgical closure.82, 173, 174 A subaortic ridge is particularly likely to develop in patients in whom adherence of the septal leaflet of the tricuspid valve is the mechanism of closure of the ventricular septal defect.82 Although rare, fixed subaortic stenosis has also bee seen following repair of tetralogy of Fallot.175–179
Surgical intervention for the long-segment and diffuse forms of stenosis have proved much more problematic. The type of surgical procedures discussed for the fibrous shelf only rarely relieve the pressure gradient, and most patients were left with residual and important obstruction, as well as the substrate for continued damage to the aortic valve.156 Because of this, a number of procedures have been developed specifically to address long-segment stenosis. Some patients were treated by placing a conduit from the apex of the left ventricle to the aorta,179–184 but the poor fate of the conduit, the impact of the surgery on the left ventricle, and the introduction of new surgical procedures have led to the near extinction of this technique. Some still use this approach for very selected patients,184 and there is no doubt that this approach has afforded reasonable palliation. It is the operations pioneered by Konno and Misbach and their respective colleagues, and by Rastan and Koncz, and then others, which provide specific relief for the tunnel variants.185–197 The original Konno procedure did not spare the aortic valve, and indeed facilitated the positioning of an appropriately sized valvar prosthesis in the patient with a small aortic root. Variations of aortoventriculoplasty sparing the aortic valve were then introduced, leading eventually to the so-called Ross-Konno procedure.188–197 These techniques are well summarized in the third edition of Cardiac Surgery as produced by Kirklin and Barratt-Boyes.156 Surgical mortalities range from 5 to 15 percent.156 The introduction of new surgical procedures has necessitated new knowledge, particularly concerning the variations in the septal perforating arteries and the anatomy of the muscular subpulmonary infundibulum.198–200 In the aftermath of cardiac surgery, preservation of cardiac function is desirable. This is as true for subaortic stenosis as any other lesion. Chan and colleagues have assessed cardiac function after repair of the fixed forms of subaortic stenosis,201 finding that systolic function was well preserved. A restrictive pattern of left ventricular filling was common, however, and presumably reflected a response to the chronic pressure load and to surgery in the child.
As might be anticipated, surgical mortality and morbidity with these procedures continues to decline with increasing experience. In the years shortly after introduction of aortoventriculoplasty, surgical mortalities were reported in the range of 10 to 24 percent.156, 189 Already by 1989, however, De Leon et al.188 had reported no early mortality in 12 patients when employing infundibular enlargement, although two patients required pacemakers. Vouhe and Neveux191 similarly produced good early and late surgical outcomes in a series of 25 patients. Others have now reported similar series.156, 163, 194, 195, 197 Thus, using any of a variety of surgical techniques, very acceptable outcomes should now be anticipated for patients with long segment subaortic stenosis. Similar operative approaches have been reported in the dog, beginning with transventricular dilation, progressing through removal of the shelf and myomectomy, and also using conduits placed from the apex of the left ventricle to the aorta.202–205
As discussed at the beginning of this section, some are now introducing balloon dilation for relief of the short segment form of stenosis. This began with the experiences of Suarez de Lezo et al.206 and Labadibi et al.207 Most reports have been anecdotal, but some series of between 20 and 40 patients have been reported. We share the concerns stated so clearly by Ritter157 and Patel and Hijazi158 that this form of therapy is rarely, if ever, justified. Yet some advocate the procedure strongly.208 We would concede that the approach may have a place in palliating the patient so as to promote recovery of left ventricular contractile function.209 It may also be a reasonable approach in a patient with a serious co-morbid illness, where cardiopulmonary bypass is contraindicated. Balloon therapy has also already been performed in the dog.210
The developmental complex bearing Shone's name is characterized by subaortic left ventricular obstruction, coarctation of the aorta, parachute mitral valve, and the supravalvar stenosing ring of the left atrium.148 The subaortic obstruction in these patients can vary from the short-segment to the tunnel form, and thus the type of surgery needed for correction will vary from removal of the shelf and myomectomy to aortoventriculoplsty.211–214 Vogt et al.74 state that, on the basis of their experience, there is an association between the most severe expression of fixed long segment subaortic stenosis and Shone's complex. This was not the experience of Brauner et al.,213 where the short-segment form of subaortic stenosis was found in most of their patients with the Shone syndrome. In their experience, the late outcome correlated with the predominance of involvement of the mitral valve, and the degree of pulmonary hypertension.
Bolling et al.212 from Ann Arbor also reviewed the outcome of 30 consecutive patients seen with Shone's anomaly between 1966 and 1989. There were no operative deaths at the first operation. One quarter of the patients, however, died after a second operation. All operative deaths were secondary to severe disease of the mitral valve. The survivors were followed from 1 to 16 years, and there were no late or sudden deaths.
Fixed subaortic stenosis, whether encountered in man or the dog, is a curious disorder, sharing aspects of both an acquired and congenital abnormality. There is more evidence in certain breeds of dog that it is a genetic disorder, or at least reflects an important genetic influence. It was the hope of Detweiler that providing an understanding of the types and epidemiology of congenital cardiac disease in the dog might shed light on some aspects of such malformations as seen in man. With regard to fixed subaortic stenosis, the many observations made by Detweiler and his colleagues at the University of Pennsylvania have certainly begun to achieve this noble goal (Table 4).
Table 4. Comparative features of fixed subaortic stenosis in man and dog (Newfoundland).

We can conclude, therefore, that both the short-segment and tunnel forms of subaortic stenosis as seen in man and dog have both acquired and congenital features. It is our belief that an abnormal cardiac scaffold may set the stage for chronic turbulent flow of blood in the left ventricular outflow tract, promoting abnormal shear forces, and leading in turn to an abnormal fibrous response at the endothelial interface, with an increase in turnover of endothelial cells. The abnormal scaffold may include an abnormal, elongated left ventricular outflow tract with increased separation between the leaflets of the aortic and mitral valves, a steeper aortoseptal angle, a small aortic root, and abnormal mitral valvar pathology, all serving to crowd the left ventricular outflow tract. The association of the fixed forms of subaortic stenosis with coarctation of the aorta, bifoliate aortic valve, congenital mitral valvar anomalies, and ventricular septal defect suggests a congenital component to the disease process, while in the human, but particularly in pure-bred dogs, genetic influences may also be present. Progression of the disease, aortic regurgitation, and infective endocarditis are common to both human and canine forms of fixed subaortic stenosis.
There has been considerable evolution in surgical therapy, with many now favouring removal of the fibrous shelf combined with myomectomy. Important worsening of aortic regurgitation, perforation of the aortic leaflet of the mitral valve with mitral regurgitation, perforation of the ventricular septum, and development of mitral annular aneurysms are fortunately now rare complications of surgical intervention. There is, however, no consensus as to the timing of operation to prevent recurrence, or to prevent ongoing damage to the aortic valve.

The painting of Paul Pry made by Landseer, and sold for £80.

Table 1.

Gross appearance of fixed short-segment subaortic stenosis. (a) The offending subaortic tissue (*) is viewed from the left ventricle with the aortic valve intact. The subaortic stenosis forms a nearly complete ring. (b) The appearance of the subaortic obstructing tissue (*) with the left ventricle (LV) opened through the thickened aortic valve (AO) leaflets. AMVL: anterior leaflet of mitral valve; MV: mitral valve; TAV: thickened aortic valve.

Diffuse, long-segment subaortic stenosis with fibromuscular obstruction. (a) There is extensive deposition of endocardial sclerotic tissue (black asterisks) in the subaortic area, with a muscular septal bulge (white asterisks) contributing to the obstruction. Note the involvement of the aortic valve leaflet (V). (b) The right axial oblique left ventriculogram of the patient whose heart is shown in Figure 3a. The angiography suggests a subaortic chamber (*), with narrowing (white arrows) beneath it. The angiogram does not indicate the extent of endocardial thickening. (c) Aortic–mitral separation in a dog with long-segment subaortic stenosis. AO: aorta; OT: left ventricular outflow tract; arrows: denoting long-segment subaortic stenosis; LV: left ventricle; MV: mitral valve. Figure 3c modified with permission from Ref. 56.

Histopathology of offending subaortic substrate in man. (a) Histological whole-mount section of the subaortic interventricular septum in the longitudinal plane showing severe subaortic endocardial fibroelastosis (arrows). (Movat pentachrome stain). (b) Photomicrograph of subaortic region of the interventricular septum showing severe (2–3 mm in thickness) endocardial fibroelastosis that shows abundant dense collagen (green stain) but relatively little elastic fibre formation (black). The underlying myocardium shows minimal change with a band of subendo-cardial fibrosis and normal myocardium within the interventricular septum (elastic trichrome stain).

Relatively ‘discrete’ examples of fixed, short-segment subaortic stenosis as imaged from left ventriculography. (a) Retrograde left long axial oblique left ventriculogram shows a linear obstructing ‘ring’ (arrows) beneath the aortic valve. (b) A different patient whose fixed subaortic tissue (arrows) is even closer to the aortic valve.

Abnormal cardiac “scaffold” with minimal systolic pressure gradient between left ventricle and aorta. Note the elongated subaortic outflow tract (*) with aortic-mitral separation. This patient does not have an atrioventricular septal defect.

Table 2.

Table 3.

Examples of long-segment subaortic stenosis. (a) This patient has important aortic–mitral (MV) separation (white arrows), with a septal bulge (white asterisk) contributing to the tunnel (black asterisk) subaortic stenosis. (b) A different patient with recurrent and unrelieved severe tunnel (large black asterisk) subaortic stenosis following earlier transaortic approach (small black asterisks note site of aortotomy). A septal bulge contributes to the obstruction (white arrows).

Echocardiograms showing short-segment, fixed subaortic stenosis. (a) Five-chamber view shows what is considered a so-called “discrete membrane” (arrows) below the aortic valve. (b) Colour Doppler image in parasternal long axis view shows flow acceleration (asterisk) starting at the level of the offending obstructive substrate. (c) Continuous wave Doppler tracing shows increased velocity above the level of the short-segment fixed obstruction. (d) Parasternal long axis view in diastole shows a regurgitant jet (arrows) through the aortic valve. AO: aorta; LA: left atrium; LV: left ventricle.

Echocardiograms showing tunnel subaortic stenosis. (a) Parasternal long axis view shows long segment narrowing (asterisks). There is discontinuity between the mitral and aortic valve leaflets. The arrows indicate hinge points of the mitral and aortic valve leaflets. (b) Colour Doppler image in a corresponding plane shows flow acceleration through the tunnel obstruction (asterisks). Refer to Figure 8 for abbreviations.

Echocardiogram in parasternal long axis view shows subaortic stenosis secondary to accessory tissue (arrow) arising from the mitral valve. Refer to Figure 8 for abbreviations.

Rapid development of subaortic stenosis following repair of coarctation of the aorta. (a) Before repair of the coarctation when the patient was 8 weeks of age, the left ventricular outflow tract was elongated with aortic–mitral separation (white asterisks), consistent with an abnormal cardiac “scaffold”. No pressure gradient was recorded between the apex of the left ventricle and ascending aorta. (b) Following repair of the coarctation with a subclavian flap aortoplasty (short white arrows), the patient developed very severe tunnel subaortic stenosis (black arrow). This catheter study was performed when the patient was 6 months of age. The level of the aortic valve is noted with a single long white arrow. The pressure in the left ventricle distal to the fibromuscular obstruction was 252/10 mmHg and in the ascending aorta, 88/52 mmHg. Figures 11a and b modified from Ref. 131.

Table 4.