Published online by Cambridge University Press: 21 January 2005
Due to underlying cardiovascular anatomy and size, epicardial pacing may be the preferred method of pacing in small children. To assess long-term safety, we reviewed all epicardial pacemakers implanted in children between 1971 and 2001. We found that 122 patients, with a median age of 5.4 years, had a total of 181 pacemakers and 260 electrodes implanted over a total follow-up of 789 patient-years. Of the total, 12 patients died after the first implantation, with one death attributable to dysfunction of the pacemaker. Reintervention was required in 75 patients after 5.0 ± 3.2 years, due to depletion of the battery in 45 patients (60%), fracture or dysfunction of electrodes in 27 patients (36%), and infection in 3 patients (4%). In univariate analyses, risk factors for reintervention were an approach via a median sternotomy, with a relative risk of 2.3 (p = 0.0087), and an indication for pacing other than atrioventricular block, with a relative risk of 1.7 (p = 0.0314). In multivariate analyses, the approach via the median sternotomy independently predicted the need for reintervention, with a relative risk of 2.1, and 95% confidence intervals from 1.1 to 4.1 (p = 0.0256). The longevity of the second pacemaker and/or its electrode, assessed in 26 patients, was 3.7 ± 2.6 years, not shorter than the first implantation (p = 0.4037). We conclude that epicardial pacing is a reliable means of achieving permanent pacing in children, with low morbidity and mortality. A substantial proportion, nonetheless, requires reintervention within five years, warranting meticulous follow-up.
As more infants with complex congenital heart disease are successfully treated with palliative and reparative interventions, the population of children requiring permanent pacing is rapidly increasing.1 Major advances in technology and techniques for implantation have taken place over the past four decades.2–6 Although endocardial pacemakers are almost universally implanted in adults with structurally normal hearts, the optimal approach is less clearly defined in children.6, 7 Epicardial pacing is often preferred in neonates, infants, and children because of their small size, complex congenital defects, intracardiac shunts, limited vascular access, and potential for growth potential.8–10 Yet, in comparison to endocardial pacing, epicardial systems are reported to have shorter longevity, in part due to higher thresholds, exit block, and lead fractures.7, 10 Moreover, children requiring life-long pacing face the prospect of multiple reinterventions.7, 9 The purpose of our study, therefore, was to assess the long-term safety with epicardial pacing by reviewing our experience in a large cohort of children.
We included all children with permanent epicardial pacemakers implanted between June 1, 1971 and March 1, 2001 at Hôpital Sainte-Justine, Montreal, Canada, excluding those patients over 18 years, a common North American definition of adulthood. All medical charts, operative records, and outpatient visits to the pacemaker clinic were retrospectively reviewed. Data were collected on age, gender, underlying cardiac disease, surgical procedures including implantation techniques, indication for pacing, generator and lead types, location, mode of pacing, acute sensing and pacing thresholds and lead impedance, complications, timing and nature of subsequent interventions, and all major outcomes including death. Perioperative mortality was defined as death occurring within 30 days of surgery. The study was approved by the institutional review board.
Epicardial leads, either unipolar or bipolar, were implanted by standard surgical techniques with access via an anterolateral left thoracotomy, midline sternotomy (Fig. 1a), or subxiphoid approach, depending on underlying anatomy, prior surgical procedures, and whether implantation was part of a combined surgery. The ventricular lead was positioned on the anterior left ventricular or diaphragmatic right ventricular surface. The atrial lead was placed on either atrium, depending on the particular cardiac anatomy and surgical approach. Antibiotics were administered to all patients postoperatively for roughly 48 h. Electrodes were sutured to the epicardium, predominantly with Prolene (Ethicon Inc., Somerville, NJ, USA) with care to prevent dislodgment. Leads were subsequently tunneled along the rib margin to the generator implant site. Through a second incision, the pacemaker generator and surplus pacing wire were placed in the retroperitoneal space, most commonly in the left flank or posterior to rectus muscle (Fig. 1b).
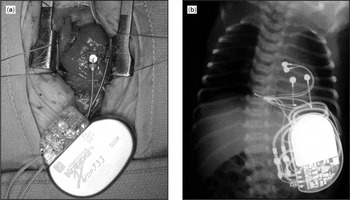
Figure 1. Implantation of a permanent epicardial pacemaker. A permanent epicardial pacemaker was implanted for congenital sinus nodal dysfunction in a 3-day-old child weighing 2.7 kg. In panel (a), the leads are positioned on left ventricular epicardial surfaces (atrial leads not yet implanted) via a median sternotomy (Note: the head is at top of the picture). The postoperative chest radiogram, panel (b), shows the position of the two epicardial leads with the generator positioned in the left flank.
Acute analyses were performed using a Medtronic pacing system analyzer (most recently PSA 5311; Medtronic, Minneapolis, MN, USA). Measurements included lead impedance at 0.5 ms/V, sensed P and R waves, and voltage pacing thresholds at a pulse width of 0.5 ms. Within the first month, at 3 months, and 6-month intervals thereafter, pacing and sensing thresholds, lead impedance, and generator voltage were routinely assessed with a Medtronic system analyzer (recently 5300; Medtronic).
Continuous variables are expressed as mean ± standard deviation. Univariate comparisons were made using an analysis of variance, Student’s t-test, the Kruskal-Wallis rank test, or Fisher’s exact test where appropriate. Curves for freedom from reintervention were plotted using the Kaplan-Meier method, and analyzed by the log-rank test. Censoring occurred in the event of placement of endocardial leads, or loss to follow-up. Cox proportional hazards models were used to evaluate unadjusted univariate and adjusted multivariate predictors of reintervention. Estimates of relative risk, along with 95% confidence intervals, were obtained from Cox models. Two-sided p values of <0.05 were considered to indicate statistical significance. All analyses were performed using SAS software Version 8 (SAS Institute, Cary, NC, USA).
During this 30-year period, we implanted 181 generators in 122 patients, using 260 epicardial leads, with a total of 242 surgical interventions. The median age at initial implantation was 5.4 years, with a range from 1 day to 18 years, and 57% were male. The mean duration of follow-up was 6.4 ± 4.7 years, and ranged from 1 day to 21.2 years, totaling 789 patient-years. Censoring due to loss to follow-up occurred in 8 patients. During the perioperative period, 5 patients died (3.8%), with 4 of the 5 deaths occurring in children with recent or combined cardiac surgery for complex congenital cardiac disease. There were an additional 8 deaths during follow-up, 7 of which were prior to any surgical reintervention, and with one attributable to dysfunction of the pacemaker.
Of the 122 patients, 101 (83%) had structurally abnormal hearts, 91 (75%) of whom had surgery for congenital heart disease, as summarized in Table 1. The most common surgical procedures were the Mustard or Senning procedures for patients with concordant atrioventricular but discordant ventriculo-arterial alignments, Fontan or bi-directional Glenn for those with functionally univentricular physiology, and repair of atrioventricular and ventricular septal defects. The median time from surgery to implantation of the pacemaker was 37 days, with a range from 0 days to 16.1 years.
Table 1. Associated congenital cardiac defects.

Prior to 1986, epicardial pacing was the exclusive mode of permanent pacing available at our institution. Of the 122 patients, 42 received their pacemakers before transvenous systems were available. The remaining 80 patients with epicardial systems represent a small proportion of all pacemakers implanted since 1986. Of these patients, 47 were under 5 years of age, 37 of whom were no more than 2 years of age. In these young children with structurally normal hearts, epicardial pacemakers were largely justified on the basis of small bodily habitus. The remaining 33 patients had epicardial systems for various reasons. In 10 patients, construction of the Fontan circulation had removed transvenous access for ventricular pacing, 4 patients had prosthetic tricuspid valves, while the remaining patients had intracardiac bi-directional or right-to-left shunting, with or without prior palliative shunts.
In accordance with the guidelines of the American College of Cardiology and the American Heart Association,11 indications for permanent pacing included postoperative atrioventricular block in 50 patients (41%), congenitally complete heart block in 31 patients (26%), sinus nodal dysfunction in 26 patients (21%), to permit adequate therapy for tachyarrhythmias in 10 patients (8%), and others in 5 patients (4%). Implantation for congenital atrioventricular block occurred at a median age of 3.6 years, with a range from 1 day to 17.8 years, compared to 2.8 years, and a range from 0.1 to 17.1 years, in patients with postoperative atrioventricular block (p = 0.1905). In 50 patients, pacemakers for postoperative atrioventricular block were implanted at a median of 21 days after the initial surgery. Of these implants, 31 pacemakers (62%) were inserted prior to discharge from hospital, within one month from the cardiac surgery.
The varied techniques for implanting epicardial pacemakers reflect the complexity of our population of patients, and mounting surgical experience. Leads were implanted by a left anterolateral thoracotomy in 97 patients, including 5 redo thoracotomies, a median sternotomy in 22 patients, including 2 redo sternotomies, and other approaches in 3 patients that included access from a subxiphoid incision.
Over the 30-year course, various models of Medtronic pacemaker pulse generators and leads were used (Table 2). Overall, epicardial atrial leads were implanted in 99 patients, and ventricular leads were implanted in 119 patients. Combined surgeries for congenital cardiac defects with implantation of epicardial pacemakers were performed in 14 patients, mainly via midline sternotomies (93%) at a median age of 4.8 years, with a range from 3 days to 18.7 years. Epicardial leads were implanted exclusively on the left ventricular surface in 31 patients, and on left atrial and ventricular surfaces in 72 patients. Pacemaker generators were positioned in the left flank, using either the retroperitoneal or pre-retroperitoneal spaces, in 115 patients (94%), in the fascia of the rectus abdominis muscle in 6 patients (5%), and in the right flank in one patient (1%). The pacing mode was atrioventricular [DDD(R)] in 89 patients (73%), ventricular without atrial synchronization [VVI(R)] in 28 patients (23%), and exclusive atrial sensing and pacing [AAI(R)] in 5 patients (4%).
Table 2. Medtronic generator and lead models at first implantation.

Acute thresholds for atrial pacing and P-wave sensing, both assessed in 89 patients, were 1.4 ± 1.0 volts at 0.5 ms, and 4.1 ± 3.5 mV, respectively. Ventricular pacing, assessed in 103 patients, and thresholds for R-wave sensing, assessed in 91, were 1.2 ± 0.9 volts at 0.5 ms, and 12.9 ± 5.4 mV. Atrial and ventricular lead impedances, assessed in 89 and 91 patients, were 510 ± 256 Ω, and 623 ± 289 Ω. When compared to a mid-line sternotomy, the left lateral thoracotomy approach was associated with a lower threshold for ventricular pacing, at 1.1 ± 0.8 V compared to 1.6 ± 1.1 V (p = 0.0271), impedance at 591 ± 241 Ω versus 757 ± 409 Ω (p = 0.0252), and a lower impedance at the atrial lead, at 468 ± 184 Ω versus 662 ± 371 Ω (p = 0.0018).
Following the initial 122 implantations, 75 patients (61%) underwent a total of 118 pacemaker-related reinterventions. Of the 75 patients, 7 had procedures in adult institutions. Of the 68 with reinterventions performed in our institution, 17 were transitioned to endocardial transvenous systems, while epicardial pacing was pursued in 51 patients. Of the latter patients, 30 required a new generator after 5.1 ± 2.4 years, with a range from 0.2 to 13.2 years, 15 required a new generator and epicardial lead(s) after 3.9 ± 2.5 years, with a range from 0.3 to 9.7 years, and 6 required replacement only of epicardial leads after 0.3 ± 0.2 years, with a range from 0.02 to 0.7 years. A third intervention was required in 26 patients after 3.7 ± 2.6 years, with a range from 0.03 to 9.0 years, a fourth in 4 patients, and a fifth in 4 patients. The decision to pursue with epicardial pacing was individualized and at the discretion of the treating cardiologist and surgeon. Nevertheless, transvenous pacing was precluded in 30 of 51 patients (59%) due to unavailability of this approach prior to 1986 for 14 patients, the particular congenital anatomy for 13 patients, very small body habitus for 2 patients aged <2 years, and venous thrombosis in 1 patient.
Freedom from reintervention followed a linear course over time, with a 16.2% annual rate of reintervention (Fig. 2a). Half the patients required a repeat procedure at 5.5 years following implantation. In univariate analysis, significant risk factors for reintervention were an approach through a median sternotomy, associated with a relative risk of 2.3, and 95% confidence intervals of 1.2–4.2 (p = 0.0087), and an indication for pacing other than atrioventricular block, associated with a relative risk of 1.7 and 95% confidence intervals of 1.1–2.9 (p = 0.0314). Age at implantation, gender, decade of surgery, underlying congenital cardiac disease, impedances of the atrial and ventricular leads, thresholds for pacing and sensing, and the number of implanted leads were not associated with need for subsequent reintervention. By multivariate analyses, the only independent risk factor for reintervention was an approach through a median sternotomy, with a relative risk of 2.1, and 95% confidence intervals of 1.1–4.1 (p = 0.0256).

Figure 2. Freedom from pacemaker reintervention in the entire cohort and stratified according to the surgical approach. In panel (a), a Kaplan-Meier curve depicts overall freedom from pacemaker reintervention. Note the linear increase in pacemaker reintervention over time. In panel (b), survival free from pacemaker reintervention is shown to be superior when a lateral thoracotomy is performed when compared to a median sternotomy.
These consisted of depletion of the battery in 45 patients (60%), infection requiring removal of hardware in 3 patients (4%), and dysfunction of the lead in 27 patients (36%), including 7 with fractures, 8 with increased pacing thresholds, and 12 with under or over-sensing. Overall, the mean time to reintervention was 5.0 ± 3.2 years, but varied significantly according to the surgical indication (p < 0.0001). For example, time to reintervention averaged 5.9 ± 2.9 years, 3.7 ± 3.2 years, and 0.6 ± 0.3 years, respectively, in those with depletion of the battery, dysfunction of the leads, and infection (Fig. 3).

Figure 3. Indications for reintervention. Kaplan-Meier survival free from pacemaker reintervention curves are plotted and compared according to whether the indication for pacemaker revision was battery depletion, lead dysfunction, or infection.
Four deep infections were observed in our cohort, prompting replacement of the generator and its lead. In one case, a severe infection with an intra-abdominal abscess required laparotomy and colostomy. Inadvertent muscular or diaphragmatic stimulation occurred in 8 patients, and symptomatic pacemaker-mediated tachycardia in 2 patients.
Of fourteen late deaths, 1 was likely attributable to the pacing system. A 1.6-year-old girl with a repaired atrioventricular septal defect with common atrioventricular junction and valve complicated by postoperative atrioventricular block died suddenly 5.7 months after implantation. In retrospect, a pacemaker-sensing defect may have resulted in her death. All other deaths were attributed to the clinical progression of underlying cardiac disease.
In our institution, epicardial pacing was first performed in 1971, and was the exclusive mode of pacing until 1986, after which both endocardial and epicardial pacing were employed. Over the past three decades, more epicardial pacemakers were implanted with 20, 43 and 59 devices implanted from 1971 to 1980, 1981 to 1990, and 1991 to 2001, respectively. Given the evolution in technology over the years, we stratified our findings by decade of surgery. Median ages at implantation were 9.7, 8.3, and 1.7 years over the past three decades, from earliest to most recent, with implantation performed at significantly younger ages over time (p = 0.0001). Median time to reintervention for implants in the first, second, and third decades was 4.0, 6.4, and 5.5 years, respectively (p = 0.8913).
Increasing indications for, and implantations of, pacemakers have paralleled important advances in technology that include improvements in electrode design, along with the size, features and reliability of the pulse generator.10, 12 In the large majority of patients, endocardial pacing is now considered the method of choice. In children, this less invasive approach is likewise gaining favor.5, 13–16 Yet, conditions such as very young age, intracardiac shunting, and complex cardiovascular anatomy6, 7 may render an epicardial pacing approach more favorable, or preclude endocardial pacing altogether. In addition to requiring a thoracotomy, that may or may not be indicated as part of a combined surgical approach to address other lesions, epicardial pacemakers have been associated with poorer pacing and sensing thresholds,7, 17, 18 and consequently higher rates of failure with need for reintervention.10, 18–20 In the present study, we explored and methodically quantitated some of the issues encountered in our long-term experience with 260 epicardial leads and 181 generators implanted in 122 children during a 30-year period.
As more and more children with congenital cardiac defects were successfully treated, and guidelines evolved for permanent pacing,11 we observed a changing pattern of implantation over the years. The predominant indication for implantation was atrioventricular block (67%), including postoperative (41%) and congenital (26%) subtypes. These indications reflect the nature of our population, and are consistent with previous reports.10, 19, 21, 22 Indeed, the majority of patients had structurally abnormal hearts, with three-quarters having undergone surgical correction of underlying cardiac malformations. Postoperative atrioventricular block is a well-recognized complication of congenital cardiac surgery.19, 22 Most instances of postoperative atrioventricular block were recognized promptly and managed appropriately with cardiac pacing during the same hospitalization.
All leads were implantated epicardially, and not transmurally, an alternative technique proposed for complex cases to improve thresholds by channeling leads to the endocardial surface through the atrial and/or ventricular wall.23, 24 The preferred technique selected for epicardial implantation was a left anterior thoracotomy that offers suitable access to the left atrium and ventricle. Given that atrioventricular block was the most common indication, dual chamber pacemakers that allow a physiologic atrioventricular response were favored. Pacemakers were most commonly placed in the left flank, an approach that allows sufficient space to implant a relatively large generator in small children. The large majority of patients had acceptable thresholds for pacing and sensing at implantation for both atrial and ventricular leads. Interestingly, an approach through a median sternotomy, when compared to left lateral thoracotomy, was associated with a greater than twofold increased risk for reintervention, even after controlling for various baseline factors and underlying congenital cardiac disease. Moreover, median sternotomies were associated with significantly higher ventricular thresholds, and atrial and ventricular lead impedances. These findings may reflect the observation that median sternotomies are associated with a greater extent of damage to the epicardial wall, resulting in relatively more fibrosis, dense scarring, adhesions, and inflammation.9, 22, 25
More than three-fifths of our patients required at least one reintervention. The time to reintervention of 5.0 ± 3.2 years compares favorably to other series assessing epicardial pacemakers,7, 19 as well as endocardial systems inserted in similar populations of children.9, 15 While depletion of the battery occurred after an average of 5.9 years after implantation, improved longevity is expected to ensue from technologic advances, including the use of steroid-eluted epicardial leads that result in lower pacing thresholds. Not unexpectedly, dysfunction or infection of the leads prompted earlier reintervention than depletion of the battery. The incidence of fractured leads in adults with epicardial pacing has been estimated to be 2% per patient-year.26 Most such fractures occur at sites subjected to increased stress, such as adjacent to the pulse generator or under the costal margin.1 Given that fractures are more common with epicardial pacing, some investigators have suggested that its occurrence should prompt transitioning to an endocardial pacing system unless otherwise contraindicated.13
When reintervention was necessary, the general policy adopted by our institution was to test the leads with continued use if their function was not impaired, followed by replacement of the generator. If leads required replacement, alternative approaches were considered, including re-implantation of epicardial leads, or replacement with a transvenous pacemaker system if not contraindicated. The rationale in support of maintaining epicardial leads was the preservation of venous access for later use in patients requiring life-long devices. Despite the numerous advantages of endocardial pacing systems, concerns remain over long-term vascular and valvar integrity with multiple transvenous leads.10, 19, 27, 28 Venous thrombosis resulting from a disproportion between the size of the vessel and the lead has been reported to occur in up to almost half the transvenous pacemakers implanted in children.1, 19
Retrospective studies have numerous potential limitations. In order to avoid potential selection bias, and allow accurate estimates of rate and risk, every child with an epicardial pacemaker implanted in our institution was included in our cohort. The main variables concerning exposure and outcome, such as surgical approach and reintervention, were objective measures with low probability for misclassification. Observation bias in assessing outcome was limited by routine at least biannual visits and minimizing losses to follow-up. Once patients reached adulthood, their care was, however, transitioned to other institutions. No comparable group with endocardial pacing group was available. Various models of both leads and generators were implanted over the span of 30 years, with all devices manufactured by the same company. An insufficient number of steroid-eluting leads were implanted to assess their impact on thresholds and the longevity of the generator. Although the population studied was heterogeneous in nature, with diverse underlying cardiac malformations and sizes of patients, every attempt was made to control for baseline imbalances in the analysis when indicated for comparisons.
We conclude that the majority of children with permanent pacemakers will require pacing systems for the rest of their lives. Our experience in a large cohort of children with long-term follow-up suggests that epicardial pacemakers are safe and reliable. In selected patients, including the very young in whom preservation of venous access is of concern, epicardial pacing should be considered a reasonable alternative. A substantial proportion of patients with epicardial pacemakers do, however, require reintervention within five years. Median sternotomy is a risk factor for such. Meticulous follow-up is essential to ensure adequate margins of safety for threshold, to anticipate need for changes in the generator, to screen for pacemaker malfunction, and to optimize programmable settings.
This study was supported in part by research fellowship scholarships from the Canadian Institutes of Health Research (NN and PK).

Implantation of a permanent epicardial pacemaker. A permanent epicardial pacemaker was implanted for congenital sinus nodal dysfunction in a 3-day-old child weighing 2.7 kg. In panel (a), the leads are positioned on left ventricular epicardial surfaces (atrial leads not yet implanted) via a median sternotomy (Note: the head is at top of the picture). The postoperative chest radiogram, panel (b), shows the position of the two epicardial leads with the generator positioned in the left flank.

Table 1.

Table 2.

Freedom from pacemaker reintervention in the entire cohort and stratified according to the surgical approach. In panel (a), a Kaplan-Meier curve depicts overall freedom from pacemaker reintervention. Note the linear increase in pacemaker reintervention over time. In panel (b), survival free from pacemaker reintervention is shown to be superior when a lateral thoracotomy is performed when compared to a median sternotomy.

Indications for reintervention. Kaplan-Meier survival free from pacemaker reintervention curves are plotted and compared according to whether the indication for pacemaker revision was battery depletion, lead dysfunction, or infection.