There is a heterogeneous array of symptoms associated with postural orthostatic tachycardia syndrome.Reference Johnson, Mack, Kuntz, Brands, Porter and Fischer 1 – Reference Li, Zhang, Hao, Jin and Du 3 These symptoms can be found across numerous body systems, including cardiovascular, neurologic, and gastrointestinal. They can present in many ways, such as dizziness, headaches, palpitations, nausea, gastrointestinal dysmotility, and chronic pain. Two of the most common and, often, most debilitating symptoms are fatigue and cognitive dysfunction, the latter of which is sometimes referred to as “brain fogReference Boris and Bernadzikowski 4 .” Even when dizziness, typically the most frequently seen symptom, is controlled, these symptoms can occur independently or in tandem. This can lead to an inability to perform routine activities of daily living, or to attend school or work. It also can adversely affect an individual’s ability to participate in exercise, one of the more important interventions that has been shown to reduce or eliminate symptoms of postural orthostatic tachycardia.Reference George, Bivens and Howden 5
Fatigue and brain fog can be among the most difficult symptoms to control in these patients. There have been limited studies in combined adult and paediatric patient series that assess the use of stimulant therapy (e.g. methylphenidate and mixed amphetamine salts), selective norepinephrine reuptake inhibitor therapy (atomoxetine), and (probable) monoaminergic stimulant therapy (modafinil) in the overall management of these specific symptoms.Reference Kanjwal, Saeed, Karabin, Kanjwal and Grubb 6 – Reference Kpaeyeh, Mar and Raj 8 We sought to look specifically at the efficacy of therapy for fatigue and brain fog in children with postural orthostatic tachycardia. The Children’s Hospital of Philadelphia developed a Postural Orthostatic Tachycardia Syndrome Program that opened in 2014, although one of the authors (J.R.B.) has been diagnosing and treating patients at the Children’s Hospital of Philadelphia since 2007; its affiliated database was created in 2016.
Materials and methods
The patient data in the Postural Orthostatic Tachycardia Syndrome Program at the Children’s Hospital of Philadelphia are managed in a REDCap database with demographic and clinical features of patients seen in both initial and subsequent clinic visits. The diagnosis of postural orthostatic tachycardia syndrome was made on the basis of a combination of historical symptoms indicating chronic orthostatic intolerance along with other typical symptoms, plus a heart rate increase of 30 or more beats per minute during a 10-minute standing test after supine position. Only patients aged 18 years and younger at the time of initial evaluation were evaluated in our study. Patients were included if they were clinically managed by either of the authors, or by another cardiologist at the Children’s Hospital of Philadelphia familiar with the treatment protocols in our clinic. We used the names, dates of birth, and medical record numbers from the database to identify patients in our clinical data warehouse populated by our electronic health record, Epic (Epic Systems, Verona, Wisconsin, United States of America). We extracted all medications ordered for these patients from their initial visit, spanning November 2007 to June 2016, including all doses and refills. Those medications that were not routinely used in the primary management of these patients, such as therapies for asthma, allergies, infections, and other disease processes not directly related to postural orthostatic tachycardia syndrome, were screened out of the analysis. The data were further filtered by symptom or symptom complex, based on their specific use in our programme. For example, medications used for light-headedness included fludrocortisone, midodrine, and desmopressin, per internal prescribing practices; there were no medications used for multiple symptoms. In the treatment of fatigue and cognitive dysfunction, we evaluated only those patients who were treated with methylphenidate, mixed amphetamine salts, dexmethylphenidate, lisdexamfetamine, atomoxetine, modafinil, or armodafinil. The use of a specific medication for an individual patient was considered to suggest clinical effectiveness if the same dose of that medication was ordered at least five times in chronological sequence. This was subsequently confirmed with a patient chart review ensuring documentation of successful therapy. Improvement of symptoms was evaluated and documented by the provider after clinical follow-up with the patient specifically regarding fatigue and brain fog. No validated method of quantification exists for symptoms related to postural orthostatic tachycardia syndrome. Thus, we assessed symptomatic improvement by qualitative review of patient chart notes, reflecting improvement in one or both symptoms following initiation of that therapy, as well as with maintenance of the same dose at subsequent visits. Patients were assessed at clinic visits with a standard clinic questionnaire that was used at all subsequent follow-up evaluations, querying the presence or absence of specific symptoms, and adding qualitative statements, as appropriate – e.g. partial improvement, specific side effects, and so on. If another provider ordered one of these specific medications for a patient for a different aetiology, these data were included if it was reported that fatigue and/or cognitive dysfunction was improved on that medication. Patients with only a single medication event with no refills or other medications used for fatigue or cognitive dysfunction were excluded. Therapy was initiated with the lowest medication dose necessary to control symptoms. Doses were subsequently titrated to maximum therapeutic effect, as tolerated or until side effects required either discontinuation of the medication or a decrease in dose. If the patient acknowledged improvement in symptoms, the medications were continued at that dose without change. If after initial improvement in symptoms with a lower dose fatigue and/or cognitive dysfunction worsened, the dose was subsequently increased. Successful therapy would be defined by at least five consecutive prescriptions of the higher dose, if achieved. Patients with partial improvement in symptoms at a specific dose but with intolerable side effects at higher doses and subsequent decrease to the lower dose were also included, as they still reported reduction of symptoms. Individual stimulants – e.g. methylphenidate, mixed amphetamine salts, and so on – were not used concurrently, as their mechanism of action was felt to be similar. However, modafinil and armodafinil were used in conjunction with these medications to attempt to potentiate the efficacy of the stimulant. If the patient had concerning side effects, such as mood changes, with one medication in a class such as stimulants, no further medications in that class were used and a different class was used – e.g. a non-stimulant such as atomoxetine.
If a specific therapy did not clinically improve symptoms, or caused intolerable side effects, the medication was discontinued. The use and the percentage of successful therapies by medication were calculated. These data were further evaluated by gender. Statistical analysis was performed using Microsoft Excel plus the website, Social Science Statistics (http://www.socscistatistics.com/Default.aspx). t-Test analysis was used to assess for differences between means in categorical variables, and χ2 test was used to assess for differences between specific categorical variables. The level of significance was set at p<0.05. A waiver of consent was granted by the Children’s Hospital of Philadelphia Institutional Review Board, as this was a review of data collected through the electronic health record for routine clinical management with intent to treat, and it was a retrospective assessment of data for which it would have been impracticable to obtain consent.
Results
A total of 722 patients with postural orthostatic tachycardia syndrome were diagnosed and treated between November 2007 and June 2016, and entered into the database for study. In all, 98% (n=708) of the patients in the database met inclusion criteria of being 18 years or younger at the time of initial evaluation. Females accounted for 77.5% of the study population (Table 1). Medications used for fatigue and/or cognitive dysfunction are listed in Table 2, including dosing range and side effects. While the side effect profiles are common across all medications with the exception of atomoxetine, there was significant patient to patient variability in tolerance of individual therapies. A total of 517 patients were chronically treated for fatigue and/or cognitive dysfunction, accounting for 73% of all patients in the paediatric postural orthostatic tachycardia syndrome database, excluding patients who received a single dose of a single therapeutic agent. The distribution of individual medications is shown in Table 3, with the majority of patients being treated with methylphenidate, followed by mixed amphetamine salts and atomoxetine, in decreasing frequency of use. Efficacy, as individual therapies and as an overall therapeutic category, is shown in Table 4. All therapies combined demonstrated a 68.8% success rate in treating fatigue and/or cognitive dysfunction. There was no difference seen between female or male patients, except with regard to dexmethylphenidate. Table 5 demonstrates the median number of medications used to achieve improvement of fatigue and/or cognitive dysfunction. Patients typically required a median of two different medications – failure because of the lack of therapeutic effect or intolerable side effects – before achieving effective clinical improvement (Fig. 1). There was no significant difference in the number of medications needed between male or female patients. Side effects for all therapies were similar, including stimulant and monoaminergic medication classes. They included decreased appetite, stomach discomfort, sleep disturbance, irritability or mood changes, worsening headache, and tachycardia. Patients who did not tolerate atomoxetine typically demonstrated mood changes, but did not present the other common side effects.

Figure 1 Number of medications used per patient.
Table 1 Patient profile.
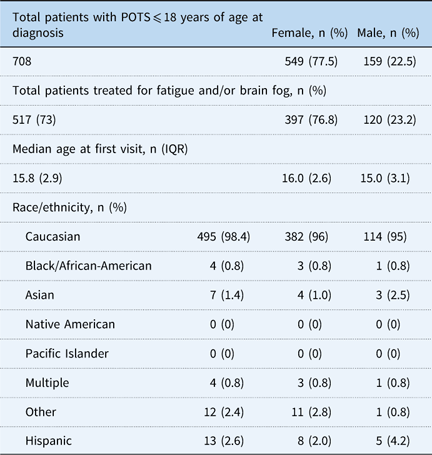
POTS=postural orthostatic tachycardia syndrome
Percentages for race may add up to more than 100%, owing to people claiming more than one race
Table 2 Medications used for management of fatigue and/or cognitive dysfunction.
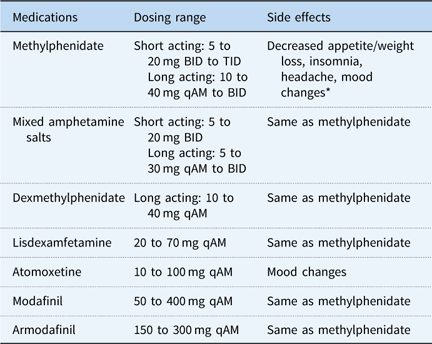
BID=twice daily; qAM=every morning; TID=three times daily
* Mood changes include irritability, anxiety, or depression
Table 3 Use of medications for fatigue and/or cognitive dysfunction.

Percentages for individual medications are for the percentage of patients in that gender treated with that specific therapy
Table 4 Therapeutic efficacy rates.
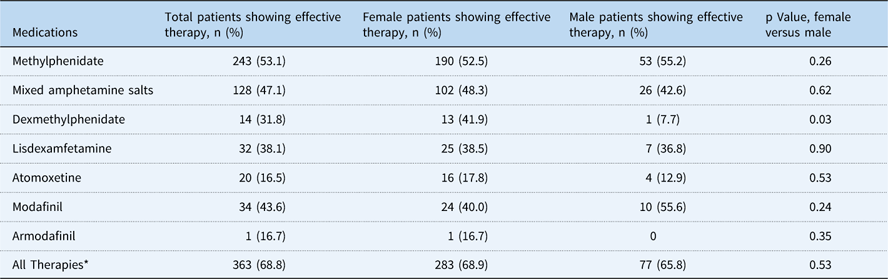
* Totals for successful use of all therapies may not equal the totals of successful use of individual therapies, as different medications may have been used in tandem
Table 5 Mean number of medications used per patient.

Discussion
The therapeutic approach to the management of postural orthostatic tachycardia syndrome is multimodal. It includes an array of nonpharmacologic interventions, including increased amounts of hydration and sodium intake, effective sleep hygiene, elevating the head of the bed, use of compression stockings, and therapeutic exercise regimens.Reference George, Bivens and Howden 5 , Reference Benarroch 9 However, even this multi-modality approach is often insufficient to adequately control patient symptoms, thus necessitating the use of medications as adjunctive therapy. Fatigue and cognitive dysfunction associated with this disorder are common complaints in patients,Reference Karas, Grubb, Boehm and Kip 10 – Reference Kizibash, Ahrens and Bruce 12 who frequently report that these symptoms specifically contribute to a significant lack of well-being and disability.
In our clinic, we use numerous nonpharmacologic and pharmacologic interventions in the management and ultimate resolution of postural orthostatic tachycardia syndrome. However, we did not see significant improvement in fatigue and brain fog until we started using classes of medications typically associated with the treatment of attention deficit disorder.Reference Sharma and Couture 13 This includes the stimulants, such as methylphenidate and mixed amphetamine salts plus their derivatives, atomoxetine, a selective norepinephrine reuptake inhibitor, and the monoaminergic stimulants modafinil and armodafinil, the actual mechanism of which is unknown. Unless there was a contraindication to therapy or a history of failed prior utilisation in our current therapeutic protocols, we routinely started therapy with a short-acting preparation of methylphenidate. This approach was used because it was the least expensive therapeutic option, there were typically fewer barriers from insurance payers, and the preponderance of patients demonstrated clinical improvement. In our database analysis, methylphenidate had the highest percentage of success in clinical improvement of fatigue and cognitive dysfunction as an individual medication, at over 53%. As a class of medications, the stimulants methylphenidate, mixed amphetamine salts, dexmethylphenidate, and lisdexamfetamine demonstrated the highest degree of success of the various classes of therapies. Conversely, atomoxetine conferred a relatively low degree of success in controlling fatigue and/or brain fog, or had intolerable side effects resulting in discontinuation of therapy. Modafinil and armodafinil were less frequently used, owing to cost of therapy and poor insurance reimbursement. However, two clinical observations related to their use were identified. First, they typically worked well for the management of fatigue, but did not change patients’ perceptions of the severity of their brain fog. Second, when used in combination with a stimulant, these medications appeared to potentiate the effect of the stimulant, thus improving both symptoms while allowing a much lower dose of the stimulant to be used. This effect was beneficial in reducing side effects and increasing tolerability, when otherwise supratherapeutic doses of stimulants were needed. Although we did not quantify specific data for this observation, it is an effect that may warrant further study.
The determination as to which therapies were used, and in which order they were started, were based on our programme prescribing patterns, and specific patient medical histories. Most often methylphenidate was the first therapy. Dosing was increased, based on therapeutic effect, and was balanced by discontinuation if there were intolerable or unacceptable side effects. If methylphenidate did not produce any therapeutic benefit, had a sub-optimal therapeutic effect, or had intolerable side effects, then mixed amphetamine salts were typically the second medication used. An allergic response, emergence of tics, or onset of side effects at even minimal doses owing to either methylphenidate or mixed amphetamine salts was considered to rule out the use of both therapies owing to their common pharmacologic profiles. The decision to use the active isomers of these therapies, dexmethylphenidate or lisdexamfetamine, was made if patients required maximal dosing of methylphenidate or mixed amphetamine salts with only modest or an absence of reduction in symptoms. Switching classes to atomoxetine occurred when one or more of the stimulants produced no improvement in symptoms or caused intolerable adverse side effects. Atomoxetine would not be used if the patient was already taking a selective norepinephrine uptake inhibitor, such as bupropion. Finally, modafinil or armodafinil would be used if prior medication trials demonstrated therapeutic failure or side effects. With regard to side effects, we observed that methylphenidate sometimes produced more gastrointestinal discomfort that resolved once the therapy was changed to mixed amphetamine salts. We also noted that long-acting or extended-release preparations had less of an overall sense of “crashing” when therapies wore off. There was also a great deal of variation in response to medication dosing. Some patients demonstrated exquisite sensitivity to very low doses of these agents. Other patients, although they may have shown a therapeutic effect, had rapid metabolism of long-acting or extended-release formulations. Thus, the patient would experience improvement in their symptoms for only 3–4 hours, compared with an expected duration of therapy of up to nine hours. These patients often required twice-daily dosing of the long-acting formulations.
The use of the different classes of medications for the treatment of fatigue and/or cognitive dysfunction often demanded iterative therapeutic attempts. Patients typically required a median of two different individual medications before achieving therapeutic success, independent of gender. Causality for this is unclear. We routinely informed patients that, for ANY symptom, it was difficult to predict which therapies would be beneficial, and which would either not work or have unacceptable side effects. Therapy for fatigue and cognitive dysfunction was no exception. A possible aetiology may be differences in individual biochemical response to these medications. In addition, postural orthostatic tachycardia syndrome itself appears to be a common final pathway after varied biological insults or triggers,Reference Garland, Celedonio and Raj 14 and may influence how individual patients respond to medications. Variability in response to these medications is recognised among patients being treated for attention deficit disorder.Reference Sharma and Couture 13 Thus, our experience with these medications in postural orthostatic tachycardia syndrome may not be a novel or unexpected response.
We found no difference in either response to medications or in the median number of medications required for efficacy between genders except with dexmethylphenidate, which suggested that it may be more efficacious for females. However, the sample size was quite small, with only 14 patients treated, so this is probably too small a sample to be able to adequately determine significance. We did note a significant difference in the number of females who were treated with methylphenidate as compared with the males. Although more females were actually treated with methylphenidate than males, the successful use of these medications was not different between the two genders. It is unclear as to why this medication demonstrated a gender disparity in frequency of use. Although we do not have specific data, one might surmise that, with a prevalence of attention deficit-hyperactivity disorder greater in males than females,Reference Norvik, Hervas and Ralston 15 male patients could have been previously treated with methylphenidate before being evaluated in our clinic.
Although we did not use a specific rating scale or quality of life assessment for these patients, they consistently reported decreased fatigue or improved energy levels, and felt as if they were able to concentrate more effectively. This supported their ability to continue schooling or job participation, and was frequently a catalyst to be able to exercise routinely and progressively, and thus ultimately to reduce and control their other symptoms of postural orthostatic tachycardia syndrome.
As achievement of successful treatment of symptoms associated with this disorder, including fatigue and brain fog, is so varied across patients, and as the range of success with these individual medications was from 16 to 53%, it could be suggested that those medications in the lower ranges of therapeutic improvement do not demonstrate a high enough rate of success over putative placebo rates to warrant continued therapeutic trials in patients. However, it is our assessment that these results still demonstrate therapeutic successes in some patients who otherwise would have had no reduction of their symptoms. Thus, the elimination of considering these treatments may deny some patients therapeutic control of these symptoms. Ensuring a brief, but adequate, trial of these therapies would allow these patients an opportunity for success as long as prompt termination of failed or poorly tolerated medications is effectively managed.
Our study does reflect a number of limitations. The definition of therapeutic success as five or more refills of a medication as a threshold parameter has not been demonstrated to be clinically valid, although it may be reasonable at this time from a rational and intent-to-treat approach until better validated measures can be established and used. In addition, it would not be rational to continue to refill a medication that either caused intolerable side effects or demonstrated no salutary effects. The success of these therapies was subsequently confirmed by chart review to document and validate clinical improvement. Our study is retrospective in nature, and is post hoc reporting of efficacy. A stronger study would evaluate these therapies in a double-blind, placebo-controlled or cross-over manner. Further, for this analysis we were not able to use a specific scale to quantify the degree of improvement in symptoms with therapy, and thus we present qualitative data only. A quantifiable symptom scale for postural orthostatic tachycardia syndrome patients, whether adult or paediatric, does not exist at present. It is hoped that, in the future, a meaningful scale can be devised and validated to better assess the efficacy of medical, as well as non-medication-based, interventions. Absent these tools currently, we used qualitative patient reporting to assess the degree of success or failure associated with the therapeutic management of fatigue and cognitive dysfunction. Finally, we did notice rarely that patients treated with midodrine to support their blood pressure for lightheadedness and tachycardia had clinical improvement in their symptoms of fatigue and brain fog. However, this effect was not common and the large majority of these patients still required separate therapy for these symptoms. In addition, we did not see improvement in these symptoms when we used other medications that support blood pressure, such as fludrocortisone or desmopressin, to reduce lightheadedness and tachycardia. That said, we did not formally compare outcomes of patients with fatigue and cognitive dysfunction treated with versus without stimulants, atomoxetine, modafinil, and armodafinil when treated with medications that alter haemodynamic support.
In the treatment of fatigue and cognitive dysfunction associated with postural orthostatic tachycardia syndrome, the use of medications that are used for attention deficit disorder can be an effective approach in controlling these symptoms. In the future, placebo-controlled blinded trials would be needed to further characterise the response to these therapies. Having these medications in the provider’s armamentarium in the management of these patients augments the ability to help these patients return to a sense of normalcy in their life.
Acknowledgements
The authors thank Andrew Glatz, MD, MSCE, for his always helpful thoughts, edits, and insight in the creation of this manuscript. The authors would also like to acknowledge Andrea Kennedy, whose wonderful expertise created our database and helped to link it to the electronic health record.
Financial Support
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation, per the United States Department of Health and Human Services, as well as the Food and Drug Administration, and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committee of the Children’s Hospital of Philadelphia.








