Tetralogy of Fallot is the most common cyanotic congenital cardiac malformation, representing 7–10% of all congenital cardiac malformations and occurring in approximately 3 per 10 000 live births.Reference Bailliard and Anderson1 Tetralogy of Fallot contains a tetrad of anatomical anomalies first described by Etienne-Louis Fallot in 1888. These include a ventricular septal defect, an overriding of the ventricular septum by the aortic root, right ventricular outflow tract obstruction, and right ventricular hypertrophy.Reference Apitz, Webb and Redington2 Tetralogy of Fallot has a multifactorial aetiology with most cases arising sporadically with no known prenatal risk factor. Both males and females are equally affected. There is an association with genetic conditions, particularly DiGeorge Syndrome (25% will have concurrent Tetralogy of Fallot).Reference Apitz, Webb and Redington2 It can also be seen less commonly in Trisomy 21, 18 and 13.Reference Bailliard and Anderson1
Tetralogy of Fallot typically presents with cyanosis, tachypnoea upon exertion, and an ejection systolic murmur. If left untreated, children may later adopt a characteristic “squatting” position to increase pulmonary blood flow in order to relieve their symptoms.Reference Nawa, Murakami and Shiraishi3
Surgical timing is based upon the patients’ comorbidities, degree of defect severity, and clinical manifestation. In asymptomatic patients, Tetralogy of Fallot surgical repair is usually performed between 3 and 11 months of age.Reference Pigula, Khalil and Mayer4 In symptomatic patients, management involves either an immediate complete surgical repair or a temporary palliative intervention followed by a complete repair at a later stage.Reference Pigula, Khalil and Mayer4 There is current controversy regarding the preferred method of managing the symptomatic neonate with Tetralogy of Fallot.
Clinical presentation of Tetralogy of Fallot
Presentation of Tetralogy of Fallot can vary widely based upon the degree of right ventricular outflow tract obstruction. Stenosis can range from a mild degree to complete atresia of the pulmonary valve. In infants with mild to moderate right ventricular outflow tract obstruction, oxygen saturations are not markedly decreased and so these children are pink in colour.Reference Bailliard and Anderson1 Neonates often present with a harsh systolic ejection murmur upon auscultation, often detectable from day 1 of life.Reference Bailliard and Anderson1 Growth and development may not be limited in these patients.Reference Forman, Beech and Slugantz5 However, the right to left shunting of blood across the ventricular septal defect can progress to congestive cardiac failure. If left unchecked, it may lead to pulmonary hypertension.Reference Bailliard and Anderson1
In Tetralogy of Fallot with pulmonary atresia, pulmonary blood flow is supplied from either the patent ductus arteriosus or major aortopulmonary collateral arteries. Tetralogy of Fallot with pulmonary atresia typically presents in the neonatal period as the patent ductus arteriosus closes. These infants appear blue with cyanosis. In infants with this variant, the patent ductus arteriosus can be maintained with a Prostaglandin E1 infusion until definitive management can be carried out.Reference Forman, Beech and Slugantz5
Tetralogy of Fallot can also present with an absent pulmonary valve. This variant is presented with non-functional valve leaflets which will cause dilation of the pulmonary artery and its branches leading to airway compression, bronchomalacia, and respiratory insufficiency.Reference Forman, Beech and Slugantz5
Tetralogy of Fallot cases are often complicated by hypercyanotic spells, a sudden decrease in oxygen saturations triggered by acutely increased right ventricular outflow tract obstruction.Reference Bailliard and Anderson1 During these spells, the amplified pulmonary vascular resistance increases the right to left shunting of deoxygenated blood across the ventricular septal defect. Patients are commonly irritable, tachypnoeic, profoundly cyanotic, and have reduced consciousness.Reference Bailliard and Anderson1 The systolic ejection murmur is often absent during these spells. Frequent hypercyanotic spells are an indicator for urgent surgical intervention.Reference Bailliard and Anderson1
Diagnosis and investigations
Tetralogy of Fallot is now prenatally diagnosed in up to 60% of patients.Reference Hill, Block and Tanem6 In the United Kingdom, this high rate of prenatal detection is a result of the Foetal Anomaly Screening Programme. Guidelines recommend ultrasound scanning in every pregnancy to screen for foetal anomalies between 18- and 20-weeks gestation. This includes echocardiographic assessment of multiple views of the heart, including the four-chamber view, right and left outflow tracts, and the ‘three vessels and trachea’ view.Reference Ward and Soothill7
Tetralogy of Fallot patients can be further investigated prenatally through pulmonary valve measurements, monitoring aorta and pulmonary trunk growth, and Doppler assessment of the great arteries.Reference Pepas, Savis and Jones8 Prenatal diagnosis and investigations facilitate early intervention: features such as reversal of flow in the arterial duct, and failure of growth of the pulmonary trunk, prompt urgent surgical intervention in the neonatal period.Reference Pepas, Savis and Jones8
Transthoracic echocardiography (Echo) is currently the most common mode of imaging in Tetralogy of Fallot patients postnatally. Although advances in Doppler Echo have reduced the requirement for cardiac catheterisation and angiography, impaired image quality sometimes limits its use.Reference Orwat, Diller and Kempny9 Thus, cardiac catheterisation is sometimes used to visualise the pulmonary arteries and major aortopulmonary collateral arteries prior to surgical intervention.Reference Handler, Ginde and Bergstrom10 Cardiac catheterisation can also be used to assess the reversibility of pulmonary hypertension and the degree of pulmonary vascular resistance. In some instances, cardiac catheterisation is diagnostic for infants with complex pulmonary atresia, when other methods of identifying major aortopulmonary collateral arteries and pulmonary artery anatomy are less reliable.Reference Wagdy11
CT and cardiovascular MRI are also used to elucidate details regarding patent ductus arteriosus-pulmonary artery morphology and coronary artery anatomy before surgical repair.Reference Handler, Ginde and Bergstrom10,Reference Wagdy11 They are advantageous in producing a detailed image of the whole heart and its vascular structures through non-invasive means. In the case of cardiovascular MRI, this is also without exposure to radiation.Reference Orwat, Diller and Kempny9,Reference Pushparajah, Duong and Mathur12 Use of cardiovascular MRI is routinely considered for procedural planning of Tetralogy of Fallot patients with complex morphology that cannot be adequately assessed by echocardiography alone. Cardiovascular MRI’s unique ability to detect myocardial fibrosis and scarring is useful in the post-operative period, particularly for detecting signs of post-operative right ventricle restrictive physiology.Reference Bacha13 However, the routine use of cardiovascular MRI is limited by cost, availability, and need for anaesthesia or sedation.Reference Apostolopoulou, Manginas and Kelekis14
Management options
Palliation
There are a number of palliative options for cyanotic Tetralogy of Fallot neonates that augment their pulmonary blood flow.Reference Glatz15 These include the modified Blalock-Taussig shunt, ductal stent, balloon pulmonary valvuloplasty, and right ventricular outflow tract stent. The modified version of the Blalock-Taussig shunt entails the insertion of a graft which connects the subclavian artery to the pulmonary artery.Reference Muralidhar16 In cases of duct-dependent Tetralogy of Fallot with pulmonary atresia, a stent is introduced into the patent ductus arteriosus, adopting either the femoral artery or the transvenous approach depending on the specific anatomy of the duct.Reference Alwi17 A pulmonary balloon dilation is carried out for patients with valve stenosis of the pulmonary artery.Reference Kumar, Remadevi and Vaidyanathan18 The newest procedure is the right ventricular outflow tract stent.Reference Dohlen, Chaturvedi and Benson19 In this procedure, which is commonly transfemoral, the pulmonary valve is balloon dilated after which a pre-mounted coronary stent is implanted across the pulmonary valve.Reference Dohlen, Chaturvedi and Benson19 Simplified illustrations of these palliative techniques are illustrated in Figure 1.
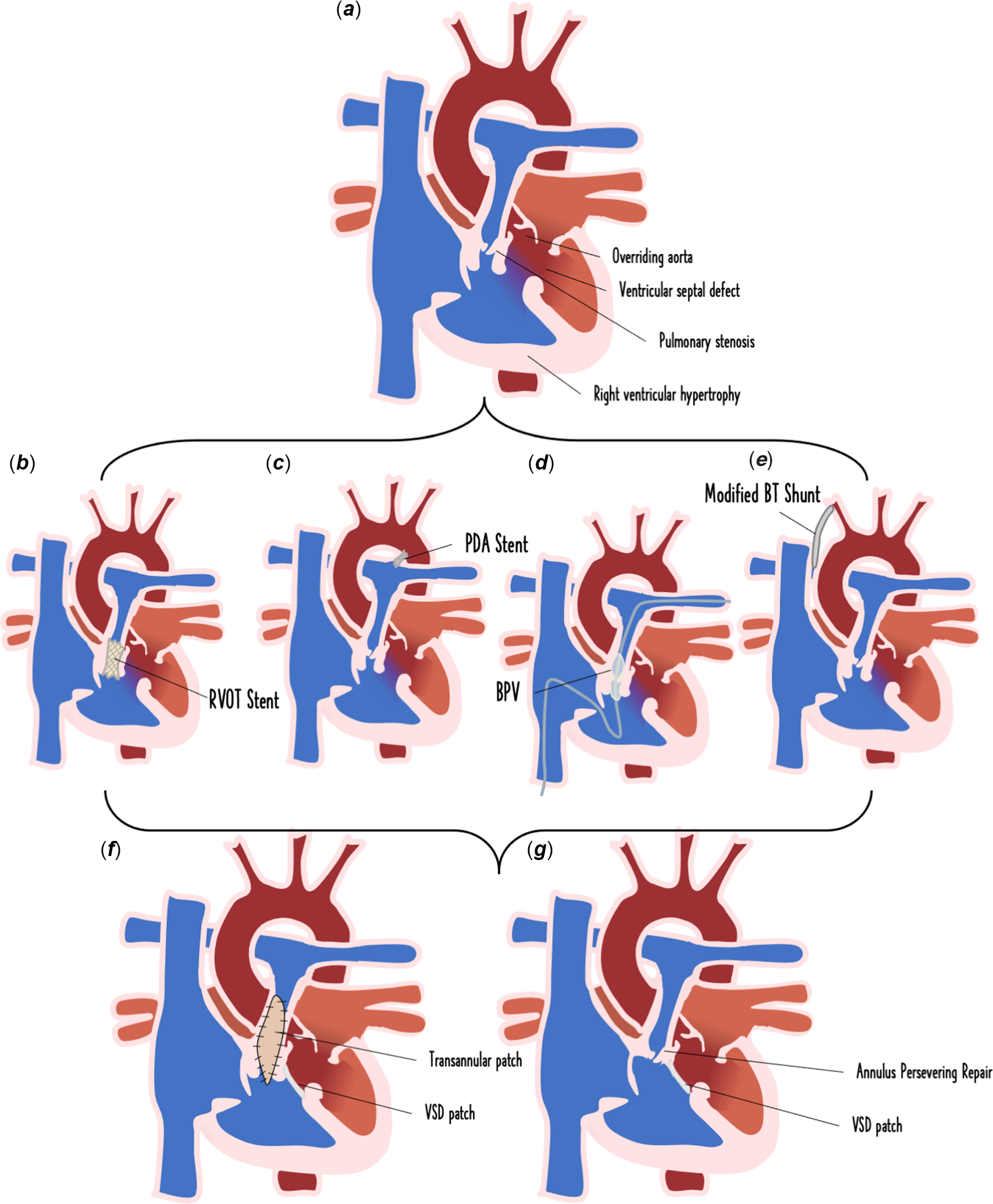
Figure 1. Palliative intervention options for the symptomatic neonate with Tetralogy of Fallot. Symptomatic neonate with Tetralogy of Fallot demonstrating tetrad of malformations: pulmonary stenosis, ventricular septal defect, overriding aorta, right ventricular hypertrophy (a), right ventricular outflow tract (RVOT) stent palliation (b), ductal stent palliation (c), balloon pulmonary valvuloplasty palliation (d), and modified Blalock-Taussig (BT) shunt surgical palliation (e), afterwards patients undergo complete surgical repair which can involves a ventricular septal defect (VSD) patch with either the transannular patch approach (f) or an annulus-preserving repair (g).
Some studies favour use of palliative intervention for symptomatic neonates with Tetralogy of Fallot over neonatal surgical repair.Reference Seddio, Migliazza and Borghi20,Reference Fraser, McKenzie and Cooley21 The palliative approach does not reduce the success of a definitive repair at a later stage.Reference Seddio, Migliazza and Borghi20,Reference Fraser, McKenzie and Cooley21 The sub-group of Tetralogy of Fallot patients in which the two-stage approach is preferred is those suffering from hypercyanotic spells, born premature, weighing <4 kg, or is <3 months of age at intervention. Palliation is also preferred in patients with anatomical variants such as atrio-ventricular septal defect or pulmonary atresia, a pulmonary artery z-score <−2, or extracardiac conditions such as necrotising enterocolitis, tracheoesophageal fistula, sepsis, or respiratory compromise that can increase the risks of single stage repair.Reference Fraser, McKenzie and Cooley21–Reference Sandoval, Chaturvedi and Benson24 The majority of patients who have a palliation procedure undergo definitive repair by 6 months of age.Reference Sandoval, Chaturvedi and Benson24
Right ventricular outflow tract stent
Right ventricular outflow tract stenting is now seen as the first-line option for Tetralogy of Fallot palliation by leading centres globally in comparison to the modified Blalock-Taussig shunt alternative.Reference McGovern, Morgan and Oslizlok23–Reference Quandt, Ramchandani and Stickley25 It is preferred due to higher morbidity (such as pulmonary stenosis, pulmonary congestion requiring diuretic use, seroma formation, or Horner’s syndrome) associated with Blalock-Taussig shunt operation.Reference Ross, Costello and Backer26,Reference Gladman, McCrindle and Williams27 Quandt et alReference Quandt, Ramchandani and Stickley25 also reported superior pulmonary artery growth in their right ventricular outflow tract stent patients in comparison to their Blalock-Taussig shunt patients.Reference Quandt, Ramchandani and Stickley25
Carrying out a right ventricular outflow tract stent procedure can be difficult in patients with a short infundibulum, right ventricular outflow tract muscular atresia, or non-confluent central pulmonary arteries.Reference Sandoval, Chaturvedi and Benson24,Reference Quandt, Ramchandani and Stickley25 These patients require an alternative form of palliation such as a Blalock-Taussig shunt, or an early primary repair. Early primary repair is also preferred to right ventricular outflow tract stenting in patients with potential for pulmonary valve preservation.Reference Sandoval, Chaturvedi and Benson24 Outcomes of palliative intervention are summarised in Table 1.
Table 1. Outcomes of palliative intervention

PICU, Paediatric ICU; RVOT, right ventricular outflow tract; PS, Pulmonary Stenosis; PA, Pulmonary Atresia; LOS, length of stay; DS, Ductal stents; RV, right ventricle.
Right ventricular outflow tract stenting is an effective method of palliation for Tetralogy of Fallot patients, with reported success rates as high as 93.6%.Reference Ghaderian, Ahmadi and Sabri28 Oxygen saturations and pulmonary artery z-scores are consistently increased after right ventricular outflow tract stenting. Oxygen saturations increased by 20.1% across 10 studies,Reference Ghaderian, Ahmadi and Sabri28 and pulmonary artery z-score increased from −2.68 to −0.92 across one cohort.Reference McGovern, Morgan and Oslizlok23
The procedural death rate of right ventricular outflow tract stenting is 3.7% across 10 studies.Reference Ghaderian, Ahmadi and Sabri28 Paediatric ICU length of stay ranges from 24 to 120 hours.Reference Ghaderian, Ahmadi and Sabri28 Re-intervention rates in right ventricular outflow tract stent patients are significant, with one trial reporting up to 54% of right ventricular outflow tract stent patients require a re-intervention before complete repair.Reference Sandoval, Chaturvedi and Benson24 Common re-interventions include repeat dilatation of the right ventricular outflow tract stent, or implantation of a second or third stent.Reference Sandoval, Chaturvedi and Benson24
During the subsequent complete repair, 95/265 (36%) of right ventricular outflow tract patients require a transannular patch.Reference Ghaderian, Ahmadi and Sabri28 Fewer patients with staged repair require a transannular patch compared to primary repair.Reference Kanter, Kogon and Kirshbom29 Cardiopulmonary bypass time for complete repair ranges from 95 to 165 minutes in patients who first have right ventricular outflow tract stent palliation. One study found that average cardiopulmonary bypass time is longer in patients with right ventricular outflow tract stent palliation compared to those with primary repair;Reference Sandoval, Chaturvedi and Benson24 however, in another study, this difference lacked statistical significance.Reference Quandt, Ramchandani and Stickley25 Sandoval et alReference Sandoval, Chaturvedi and Benson24 found that no patients with a right ventricular outflow tract stent had pulmonary valve preservation after complete repair, compared to 41% of early repair patients. Accordingly, right ventricular outflow tract stent palliation is usually not considered in patients with potential for pulmonary valve preservation.Reference Sandoval, Chaturvedi and Benson24
One study found average post-operative Paediatric ICU length of stay after complete repair was an average of 5 days in right ventricular outflow tract stent palliation patients. This was slightly shorter than early repair groups (Pulmonary Stenosis = 6, Pulmonary Atresia = 7). Average post-operative hospital length of stay was reported as an average of 11 days for the stent group, also slightly shorter than early repair groups (Pulmonary Stenosis = 13, Pulmonary Atresia = 14).Reference Sandoval, Chaturvedi and Benson24
There are varied medium-term outcomes reported for Tetralogy of Fallot patients with right ventricular outflow tract stent palliation before complete repair. One study found after 2.1 median years, 40% of patients have persistently elevated right ventricle pressures and 40% require multiple re-interventions such as left pulmonary artery dilatations to increase pulmonary blood flow.Reference Dohlen, Chaturvedi and Benson19 Another study similarly reports high re-intervention rates (25%), but no deaths by 3.2 median years.Reference Sandoval, Chaturvedi and Benson24 There is limited evidence describing long-term outcomes in Tetralogy of Fallot patients with stent palliation.
Ductal stent
Ductal stents are typically performed for duct-dependent lesions with compromised patency of right ventricular outflow tract.Reference Alwi17,Reference Sandoval, Chaturvedi and Benson24 There are various approaches to inserting this type of stent depending on the relationship of the patent ductus arteriosus to aorta.Reference Alwi17 An advantage of ductal stent is a reduction of complications commonly associated with the Blalock-Taussig shunts.Reference Alsagheir, Koziarz and Makhdoum30
A meta-analysisReference Alsagheir, Koziarz and Makhdoum30 comparing ductal stent to Blalock-Taussig shunt in neonates with duct-dependent lesions (not exclusively Tetralogy of Fallot) found ductal stent to be superior to Blalock-Taussig shunt in terms of risk for procedural-related complications. Ductal stent patients had complications such as arrhythmia (3.8%), vascular injury during access (9.4%), and ductal stent failure (15.7%).Reference Alsagheir, Koziarz and Makhdoum30 Median Paediatric ICU length of stay was 4.2 days, and hospital length of stay 12 days. There was no significant difference in 30-day mortality between patients who had a ductal stent and those who had a Blalock-Taussig shunt; however, ductal stent patients had better medium mortality rates.Reference Alsagheir, Koziarz and Makhdoum30 Forty-four percentage of patients who underwent ductal stent had re-interventions, including balloon angioplasty of ductal stent, re-stenting, and pulmonary valvuloplasty.Reference Alsagheir, Koziarz and Makhdoum30 Ductal stent can have negative impacts at time of complete repair, such as pulmonary artery distortion complicating surgery, and need for stent removalReference Alsagheir, Koziarz and Makhdoum30 and should thus only be undertaken after careful consideration. Further studies looking at both short- and long-term outcomes of ductal stent in exclusive Tetralogy of Fallot cohorts would be beneficial.
Balloon pulmonary valvuloplasty
Balloon pulmonary valvuloplasty is best suited for patients with pulmonary artery valvular stenosis rather than infundibular obstruction.Reference Kim, Ban and Lee31 In this cohort, it can be used to delay early surgical repair in symptomatic neonates by enhancing blood flow through the pulmonary arteries, promoting pulmonary annulus growth and increasing oxygen saturations.Reference Kim, Ban and Lee31 However, balloon pulmonary valvuloplasty is less effective in cases with an hypertrophied infundibulumReference Wu, Wang and Lee32, and these patients often require early re-intervention with a Blalock-Taussig shunt before definitive repair.Reference Muneuchi, Watanabe and Sugitani33
After balloon pulmonary valvuloplasty, studies report an increase in pulmonary artery z-scores and oxygen saturations. One recent study reports a z-score increase from −3.56 to −1.82 after balloon pulmonary valvuloplasty.Reference Muneuchi, Watanabe and Sugitani33 Another study states that the average oxygen saturations increased from 63 to 87% (+24%).Reference Wu, Wang and Lee32
Transannular patching is required less often after balloon pulmonary valvuloplasty when compared to Blalock-Taussig shunt.Reference Kim, Ban and Lee31 One study reported only 29% of patients with balloon pulmonary valvuloplasty required transannular patch; this is compared to 90% with Blalock-Taussig shunt.Reference Kim, Ban and Lee31 This is because the balloon pulmonary valvuloplasty technique successfully promotes pulmonary annular growth in most patients.Reference Ducas, Harris and Labos34 Muneuchi et alReference Muneuchi, Watanabe and Sugitani33 found that 48% (15/31) of patients who had a balloon pulmonary valvuloplasty still required another palliative surgical repair, an aortopulmonary shunt, before complete repair. The patients who required an aortopulmonary shunt before complete repair had greater infundibular obstruction.Reference Muneuchi, Watanabe and Sugitani33
Complete repair
The surgical management of Tetralogy of Fallot has had significant advances; the majority of centres now opt for a transatrial or transatrial–transpulmonary approach with excellent long-term results.Reference Luijten, Van den Bosch and Duppen35 This is an improvement from the original operation requiring a ventriculotomy. A ventriculotomy required a longer incision through the ventricle, pre-disposing the patient to a higher rate of complications.Reference Padalino, Vida and Stellin36
The two main objectives for open repair are closing the ventricular septal defect and correcting the right ventricular outflow tract obstruction. Once the ventricular septal defect has been patched, the aorta will no longer receive deoxygenated blood from the right ventricle, removing the overriding aorta.Reference Karl37 Once the right ventricular outflow tract obstruction is repaired, afterload on the right ventricle reduces, leading to lower contractility and reducing right ventricular hypertrophy.Reference Spinale38 A popular technique to repair the ventricular septal defect involves a polytetrafluoroethylene patch using a single continuous stitch through the tricuspid valve.Reference Menaissy, Omar and Mofreh39
The need for neonatal primary repair is dependent on the severity of the condition. If the patient is experiencing end organ damage from cyanosis, increased cyanotic episodes, pulmonary valve atresia, or requiring administration of PGE1, neonatal repair is preferred over a delayed repair.Reference Karl37,Reference Steiner, Tang and Gossett40 Neonatal repair more often requires transannular patch compared to a delayed repair.Reference Loomba, Buelow and Woods41 Indications are summarised in Table 2.
Table 2. Indications for palliation versus neonatal open repair
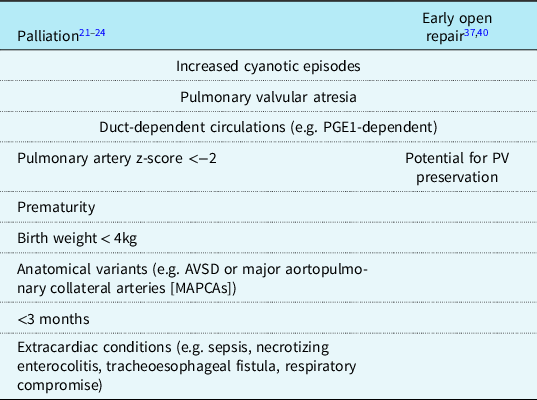
PV, pulmonary valve.
Transannular patch versus annulus preserving repair
Repair of the right ventricular outflow tract obstruction involves either a transannular patch or annulus-preserving repair.Reference Ducas, Harris and Labos34,Reference Sharkey and Sharma42,Reference Jeon, Kim and Kwon43 The optimum treatment for Tetralogy of Fallot involves limiting both residual post-operative pulmonary regurgitation and pulmonary stenosis, so selecting the correct procedure is imperative.Reference Jeon, Kim and Kwon43,Reference Yun44 Many valve-sparing surgical techniques have been described, such as balloon pulmonary valvuloplasty, pulmonary cusp patch reconstruction, pulmonary valve commissurotomy +/− rigid bougie dilatation, and complete resection of sub-valvular and supra-valvular areas of obstruction.Reference Bacha13 An annulus-preserving repair is preferable over a transannular patch due to reduced incidence of future valve replacement, lower rates of severe right ventricle dilation and pulmonary regurgitation, and better right ventricle function at first adult follow-up.Reference Ducas, Harris and Labos34 Annulus-preserving repairs are described as up to 6 times less likely than transannular patch to undergo pulmonary valve replacement indicating that transannular patch should only be done if necessary.Reference Ducas, Harris and Labos34
A transannular patch procedure is commonly considered if the pulmonary annulus does not reach a threshold size,Reference Khan, Drury and Stickley45 a finding more frequent in neonatal repairs compared to later repairs. The long-term complications of arrhythmias and ventricular dysfunction mean that transannular patch are avoided when possible.Reference van der Ven, van den Bosch and Bogers46 Moreover, abnormal coronary arteries or a distinctly small pulmonary artery may require a conduit rather than a transannular patch.Reference Khan, Drury and Stickley45
Due to anatomical variation of pulmonary valves in Tetralogy of Fallot, it is not always possible to preserve the pulmonary valve. One study discovered 10% of their patient cohort had monocuspid valves which are not suitable for pulmonary valve preservation.Reference Vida, Angelini and Guariento47 Promisingly, 90% of their patient cohort had bicuspid or tricuspid pulmonary valves. Of those 90%, in 48.7%, they were thickened and dysplastic and not able to be preserved, leaving 56% of the original cohort morphologically suitable for pulmonary valve preservation.Reference Vida, Angelini and Guariento47
Pulmonary valve replacement
Pulmonary valve replacement is indicated in patients with significant pulmonary regurgitation; other indications include presence of heart failure or new arrhythmias due to right ventricle dilatation.Reference Weinberg and McElhinney48 Pulmonary valve replacement is usually given in adulthood (ranging from 12 to 31 years after complete repair).Reference Cocomello, Meloni and Rapetto49 Interestingly, recent evidence suggests that pulmonary valve replacement does not have significant clinical outcomes in comparison to conservative management of pulmonary regurgitation. Both a meta-analysis and multicentre observational study found that pulmonary valve replacement did not significantly impact right ventricle ejection fraction, atrial tachycardia, sustained ventricular tachycardia, heart failure, or death.Reference Mongeon, Ben Ali and Khairy50,Reference Bokma, Geva and Sleeper51 Instead, the meta-analysis only found significant impact on NYHA class and right ventricle volumes.Reference Mongeon, Ben Ali and Khairy50 Both studies highlighted a need for more evidence comparing outcomes of pulmonary valve replacement with conservative management.
Immediate post-operative outcomes
Evidence highlights that early repair increases ICU and hospital length of stay, and risk of post-operative cardiac events.Reference Jeon, Kim and Kwon43,Reference Bailey, Elci and Mascio52 Bailey et alReference Bailey, Elci and Mascio52 report that ICU length of stay was increased by 90% to an average of 6 days in those having an early primary repair in comparison to a staged approach.Reference Bailey, Elci and Mascio52 One study reported infants >90 days old undergoing complete repair had both a lower rate of post-operative complications and a shorter hospital length of stay compared to neonates.Reference Yang, Wen and Tao53 Moreover, neonatal Tetralogy of Fallot repairs had the highest mortality rate (9.82%) compared to those >180 days old (0.85%) with the same open repair procedure.Reference Yang, Wen and Tao53
In comparison to staged repair, a retrospective study analysing 2,363 patients noted a higher risk of mortality in those with complete neonatal repair during hospital stay, at 30-day follow-up, and 2-year follow-up.Reference Savla, Faerber and Huang54 Patients with neonatal repair had increased cardiac complications (ECMO, Pleural effusion, need for CPR) and longer hospital stay.Reference Savla, Faerber and Huang54 This study also adjusted for confounders, such as patient, hospital, and systematic factors.
There are common complications immediately following a complete neonatal Tetralogy of Fallot repair that require further intervention, investigation, and monitoring. These complications include: residual ventricular septal defect, residual and persistent right ventricular outflow tract obstruction, arrhythmias (ventricular tachycardia or atrial fibrillation/flutter), right bundle branch block, or sudden cardiac death.Reference Townsley, Windsor and Briston55 Other commonly reported immediate post-operative complications include pericardial effusion requiring draining, pleural effusion requiring drainage, chylothorax, bleeding requiring re-operation, superficial wound infection, and junctional ectopic tachycardia.Reference Mouws, de Groot and van de Woestijne56 In patients with transannular patch repair, right ventricle restrictive physiology is commonly reported due to poor diastolic relaxation, which can contribute to a low cardiac output state.Reference Norgård, Gatzoulis and Moraes57 This is commonly managed by optimising the ventricular preload through fluid resuscitation or by mechanical circulatory support if necessary.Reference Chandler and Kirsch58 While timing of the repair is a significant risk factor for increased mortality and morbidity in Tetralogy of Fallot patients, frequency of complications also depends largely on the severity of Tetralogy of Fallot. Important factors include pre-operative size of the pulmonary valve and pulmonary arteries, right ventricular – pulmonary artery pressure gradient, and oxygen saturation.Reference van der Ven, van den Bosch and Bogers46
Long-term complications
Long-term survival after Tetralogy of Fallot repair is generally very good, with >90% of patients surviving after close to 30-year follow-up.Reference Luijten, Van den Bosch and Duppen35,Reference Smith, McCracken and Thomas59 While staged repair increased risk of mortality in the first 6 years following repair, there was no significant difference after this period.Reference Smith, McCracken and Thomas59 Pulmonary regurgitation is the most common long-term complication in patients who survive Tetralogy of Fallot repair; 20 years after complete repair, up to 64% of patients have pulmonary regurgitation.Reference Hoashi, Kagisaki and Meng60 Risk factors for pulmonary regurgitation include a larger transannular patch and prolonged post-operative ventilator support.Reference Kim, Sung and Kim61 Pulmonary regurgitation contributes to right ventricle dilatation in Tetralogy of Fallot patients, which reduces exercise tolerance and increases risk of right ventricle dysfunction, heart failure, and sudden cardiac death.Reference Hickey, Veldtman and Bradley62
Smith et alReference Smith, McCracken and Thomas59 also report the frequency of repaired Tetralogy of Fallot complications, such as arrhythmia (n = 12 [8.3%]), cardiac arrest (n = 34 [23.5%]), and congestive heart failure (n = 26 [17.9%]).Reference Smith, McCracken and Thomas59 Factors contributing to heart failure in repaired Tetralogy of Fallot include damage to the myocardium (due to multiple cardiac surgeries, insufficient myocardial protection during cardiopulmonary bypass, or long-standing palliative shunts), chronic volume overload (due to pulmonary regurgitation), and size of transannular patch (which can cause large segments of the right ventricular outflow tract to be akinetic or dyskinetic).Reference Wald, Marie Valente and Marelli63 Outcomes of early primary surgical repair are summarised in Table 3.
Table 3. Outcomes of early primary repair

PICU, Paediatric ICU; RVOTO, right ventricular outflow tract obstruction; RBBB, right bundle branch block; TOF, Tetralogy of Fallot; RV, right ventricle.
Future innovations
New methods are constantly being explored to improve outcomes for patients with Tetralogy of Fallot. With advancing technology within surgery, this is increasingly possible. Technology such as 3D-printing enhances both surgeons’ practical skills and their detailed knowledge of specific patient’s anatomy, which can improve procedural planning.Reference Loke, Harahsheh and Krieger64 Another innovation is the use of virtual reality for training and enhancing the surgeon’s perception of anatomy. A Cambridge hospital currently uses a number of virtual reality headsets for surgical training and preparation prior to complex surgery.Reference Moorjani, Lewis and Shah65
Other future innovations involve using new technology to intervene in Tetralogy of Fallot patients as an alternative to the traditional surgical repair. These innovations include high-frequency ultrasound to ablate muscle mass – a potential solution for infundibular stenosis with supporting evidence for use in hypertrophic cardiomyopathy.Reference Miller, Lu and Dou66 Use of new generation stents that can either mimic growth or biodegrade may alter right ventricular outflow tract stenting as a permanent alternative to surgical repair.Reference Borhani, Hassanajili and Ahmadi Tafti67 Furthermore, use of new perimembranous ventricular septal defect closure devices may provide an alternative to surgical ventricular septal defect closure.Reference Miller, Lu and Dou66 While risk of damage to the aorta or conduction problems may be higher using device ventricular septal defect closure, randomised control trials report similar results in comparison to surgical closure – however, these are often in older and larger children.Reference Miller, Lu and Dou66
Another innovation is use of percutaneous pulmonary valve implantation as an alternative to surgical pulmonary valve replacement . One model predicts that three pulmonary valve replacements would be required throughout adult Tetralogy of Fallot life: an initial percutaneous pulmonary valve implantation in young adulthood, followed by one in middle age and another closer to old age.Reference Barron and Vanderlaan68 The subsequent percutaneous pulmonary valve implantation would utilise the valve-in-valve percutaneous method, which involves inserting the valve using a catheter. This would limit patients to only one surgical intervention after Tetralogy of Fallot repair.Reference Barron and Vanderlaan68 While applying this model in real life might prove challenging, studies have established that percutaneous pulmonary valve implantation can reduce the number of surgeries in a patient with Tetralogy of Fallot.Reference Lurz, Coats and Khambadkone69 However, use of innovations which may worsen outcomes is controversial, particularly because outcomes following surgical Tetralogy of Fallot repair are generally very good.
Conclusion
Tetralogy of Fallot repair has very good outcomes overall, with >90% survival at 30 years. The ideal time for intervention is around 6 months. However, in the symptomatic neonate with insufficient pulmonary blood flow, early intervention is required. In some patients, a staged repair with stent palliation is preferable to early primary repair. This is often in patients with small size, complex anatomy, or comorbidities. Long-term survival between staged repair and primary repair appears to be similar. There is lack of studies that compares long-term survival of newer stent palliation methods to neonatal complete repair. This highlights a need for further evidence, particularly as these options become more popular in comparison to the traditional option of a Blalock-Taussig shunt.
Acknowledgements
None.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.







