Takayasu’s disease is a rare chronic vasculitis of unknown aetiology, predominantly affecting the aorta and its main branches, and the pulmonary arteries. It produces a variety of ischaemic symptoms due to stenosis and thrombosis of major arteries (Fig. 1). Acute progression of the disease can lead to destruction of the arterial media, formation of aneurysms, or arterial rupture.

Figure 1 Maximum intensity projection magnetic resonance angiogram subsequent to administration of gadolininum, demonstrating a severe long segment stenosis of the left common carotid artery involving its origin.
The first description of Takayasu’s disease was given in 1830 by Rokushu Yamamoto. He described a 45-year old man with fever, pulselessness, loss of weight, and breathlessness, who died in his 11th year of follow-up. In 1905, Mikito Takayasu, professor of ophthalmology, described a 21 year old woman with peculiar arteriovenous malformations of her optic funduses. Although Takayasu did not indicate that any other arteries were involved, in the discussion that followed, two other ophthalmologists, Onishi and Kagoshima, described similar ocular findings, along with absence of the radial pulse. In 1951, the clinical features of Takayasu’s disease were summarised in an English journal under the name of the pulseless disease.Reference Numano, Okawara, Inomata and Kobayashi1
Epidemiology
Takayasu’s disease is the third commonest vasculitis in childhood worldwide, but is relatively uncommon in Europe and North America.Reference Dillon2 In 1990, the Japanese government added Takayasu’s disease to the list of intractable diseases, with 5000 cases added to the list over the subsequent decade.Reference Numano, Okawara, Inomata and Kobayashi1 A North American study of patients in Minnesota found the incidence to be 2.6 cases per million population in each year.Reference Hall, Barr, Lie, Stanson, Kazmier and Hunder3 The true extent of the disease in the west is not known. In 2006, a pilot paediatric registry, in North America and Canada, was set up for chronic vasculitis, including Takayasu’s disease, to answer some basic questions about epidemiology, presenting and diagnostic features, and initial therapeutic approaches at the onset of disease.Reference Wilkinson, Page, Uribe, Espinosa and Cabral4
The disease commonly presents between the ages of 10 and 20 years, with three-quarters of patients presenting in this period, with a ratio of males to females of 1 to 8.5.Reference Lupi-Herrera, Sanchez-Torres, Marcushamer, Mispireta, Horowitz and Vela5 There is, nonetheless, a wide range of presenting age, with some presenting as early as 24 months.Reference Ladhani, Tulloh and Anderson6 A delay between symptoms and diagnosis of 2 to 11 years is seen in the west,Reference Lupi-Herrera, Sanchez-Torres, Marcushamer, Mispireta, Horowitz and Vela5 with greater delay observed in juveniles as opposed to adult populations.Reference Kerr, Hallahan and Giordano7 A study in IndiaReference Jain, Sharma, Singh, Bali, Kumar and Sharma8 of patients with Takayasu’s disease under 18 years of age reported a delay of only 2.5 to 5.5 months. This presumably reflects the higher incidence in the Indian sub-continent, and thus increased clinical awareness.
Pathophysiology
Takayasu’s disease can be divided into two stages, with an acute period of large vessel vasculitis, followed by fibrosis and scarring. In the acute stage, the adventitial vessels of the arterial walls become inflamed. The media is infiltrated by lymphocytes and occasional giant cells. Neovascularisation originates at the junction of the media and adventitia, and subsequently fans out to incorporate the entire media (Fig. 2). The intima becomes thickened, with depositions of mucopolysaccharides, smooth muscle cells, and fibroblasts.

Figure 2 Magnetic resonance angiogram after administration of gadolininum. Axial image at the level of the aortic arch. A thin rim of high signal can be seen posteriorly (neovascularisation within the outer layer of the aortic wall) separated from the aortic lumen by a thick area of low signal (the thickened aortic media).
In the chronic stage, the elastic tissue is replaced by fibrosis, with thickening of all three layers. There is patchy luminal narrowing, often affecting multiple sites. Macroscopically, the intima may be rigid, with a “tree bark” appearance, a feature common to many arteritides.Reference Gravanis9 Aneurysmal formation also occurs, as an abnormal response to mural stress because of inflammation, and may be exacerbated by increased volume, as with aortic regurgitation (Fig. 3).

Figure 3 Axial T1-weighted magnetic resonance image at the level of the bifurcation of the pulmonary trunk. The ascending aorta is dilated, and surrounded by an intermediate signal thickened wall.
Infection, in particular tuberculosis, has been implicated in the pathogenesis of Takayasu’s disease. One study reported caseating granulomatous lymphadenitis in over two-thirds, compared with less than one-tenth of the control population.Reference Lupi-Herrera, Sanchez-Torres, Marcushamer, Mispireta, Horowitz and Vela5 A South American study reviewed the clinical features in 26 children, and found a high frequency of positive purified protein derivative, in almost three-quarters, and lymphadenopathy in over one-third, with a histological picture of caseating granulomas, suggesting a link with atypical mycobacterial infection.Reference Morales, Pineda and Martinez-Lavin10 A retrospective study in India, found that one-fifth of patients had strongly positive skin tests for tuberculosis, and had been started on anti-tubercular therapy. Less than one-twentieth, however, had active tuberculosis, with all cases involving pulmonary infection.Reference Subramanyan, Joy and Balakrishnan11 Given the endemic nature of tuberculosis in Asia, and South America, it is unclear as to whether this represents a causal immunopathogenic relationship, or whether this is coincidence.
Further support to infection as a trigger for Takayasu’s disease is lent by immunopathologic analyses. Expression of 65 kDa heat shock protein, as well as human leucocyte antigens of the first and second classes, are enhanced in Takayasu’s arteritis lesions, supporting the pathogenic role of CD4 and CD8 T-cells.Reference Seko12
Clinical features
The clinical manifestations of Takayasu’s disease are commonly divided into early, pre-pulseless, and late pulseless phases. During the early phase, non-specific systemic symptoms and signs predominate, albeit often unrecognised. The late phase is characterised by ischaemia, and symptoms secondary to arterial occlusion. Recurrent disease often occurs in new arterial territories, with the coexistence of active and quiescent disease.
Symptoms and signs include
• Fever, breathlessness, haemoptysis, headache, dizziness, vertigo, angina, chest wall pain and claudicant pain.
• Reduced or absent pulses, resulting in discrepancies of blood pressure between limbs in over half the patients.Reference Hall, Barr, Lie, Stanson, Kazmier and Hunder3, Reference Lupi-Herrera, Sanchez-Torres, Marcushamer, Mispireta, Horowitz and Vela5, Reference Subramanyan, Joy and Balakrishnan11
• Hypertension in from one-third to three-quarters.Reference Hall, Barr, Lie, Stanson, Kazmier and Hunder3, Reference Lupi-Herrera, Sanchez-Torres, Marcushamer, Mispireta, Horowitz and Vela5, Reference Kerr, Hallahan and Giordano7 This is secondary to a number of overlapping factors. Marked narrowing of the aorta is associated with renovascular disease in up to two-thirds,Reference Hall, Barr, Lie, Stanson, Kazmier and Hunder3, Reference Lupi-Herrera, Sanchez-Torres, Marcushamer, Mispireta, Horowitz and Vela5, Reference Kerr, Hallahan and Giordano7, Reference Sharma, Rajani and Talwar13 with reduced aortic elasticity, and aortic regurgitation in around one-quarter.Reference Kerr, Hallahan and Giordano7, Reference Morales, Pineda and Martinez-Lavin10
• Vascular bruits are heard in over four-fifths,Reference Hall, Barr, Lie, Stanson, Kazmier and Hunder3, Reference Lupi-Herrera, Sanchez-Torres, Marcushamer, Mispireta, Horowitz and Vela5, Reference Kerr, Hallahan and Giordano7 mostly involving the carotid arteries, and rarely the femoral and renal arteries. Multiple bruits are heard in one-third.Reference Kerr, Hallahan and Giordano7
• The aortic regurgitation found in up to one-quarter of patients results from aortic dilation, separation of the attachments of the valvar leaflets at the sinutubular junction, and thickening of the leaflets.Reference Kerr, Hallahan and Giordano7, Reference Subramanyan, Joy and Balakrishnan11
• Congestive cardiac failure is seen in up to half the patients.Reference Lupi-Herrera, Sanchez-Torres, Marcushamer, Mispireta, Horowitz and Vela5, Reference Muranjan, Bavdekar, More, Deshmukh, Tripathi and Vaswani14 This may be related to hypertension, aortic regurgitation, and occasionally dilated cardiomyopathy.Reference Subramanyan, Joy and Balakrishnan11
• Pulmonary arterial involvement is encountered in up to five-sixths of patients (Fig. 4).Reference Sharma, Rajani and Talwar13, Reference Paul, Hernigou and Lefebvre15–Reference Yamato, Lecky, Hiramatsu and Kohda17 The lowest frequency was seen in an Indian population,Reference Sharma, Rajani and Talwar13 whereas the highest frequencies were observed in Japanese populations.Reference Yamada, Shibuya and Matsubara16–Reference Yamato, Lecky, Hiramatsu and Kohda17

Figure 4 Coronal oblique T1-weighted magnetic resonance image, demonstrating intermediate signal thickening of the aorta, arch vessels, and left pulmonary artery.
• Coronary arterial disease is found in one-tenth.Reference Matsubara, Kuwata, Nemoto, Kasuga and Numano18 This is usually observed at autopsy, since involvement is not apparent until the onset of angina, myocardial infarction, or congestive cardiac failure. Three types of disease are identified: stenosis or occlusion of the coronary arterial orifices and proximal segments of the coronary arteries; diffuse or focal coronary arteritis, which may extend diffusely to all epicardial branches, or may involve focal segments, so-called skip lesions; and coronary arterial aneurysms.
• Major neurological events occur in one-half. These can be transient ischaemic attacks, cerebral infarction, hypertensive encephalopathy, seizures,Reference Kerr, Hallahan and Giordano7 and even moyamoya phenomenon.Reference Wang19 These events are related to a combination of carotid and vertebral arterial disease, and hypertension.
• Hypertensive retinopathy is seen in almost one-third, and Takayasu’s retinopathy in one-sixth.Reference Chun, Park, Park, Chung and Lee20 Classical ophthalmic features of the disease are due to reduced ocular perfusion, which manifests as hypoxic retinal changes. Occlusion of branches of the retinal artery has also been described.Reference Kaushik, Gupta, Gupta, Jain and Lal21
• Raynaud’s syndrome, also seen in one-sixth, is directly related to involvement of large arteries. Other dermal lesions, seen in one-eighth, include erythematous nodules on the legs, ulceration, malar flush, urticaria, and livedo reticularis.Reference Frances, Boisnic and Bletry22
Diagnostic criterions
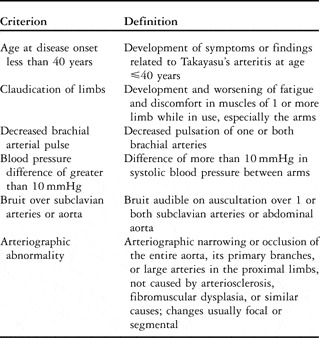
A diagnosis of Takayasu’s disease requires that at least 3 of 6 criterions be met as outlined by The American College of Rheumatology (Table 1). The presence of 3 or more of these 6 criterions demonstrates a sensitivity of 90.5%, and a specificity of 97.8%.Reference Arend, Michel and Bloch23
Table 1 The American College of Rheumatology 1990 criterions for the diagnosis of Takayasu arteritis.Reference Arend, Michel and Bloch23
Prognosis
The prognosis of the disease is affected by the clinical classification (Table 2). The five-year survival rate from diagnosis is 100% for those in groups 1 and 2a, and from 70 to 80% for those in groups 2b and 3.Reference Subramanyan, Joy and Balakrishnan11, Reference Ishikawa24 Survival is worse by one-sixth in those with hypertension,Reference Mishima25 since this may cause cardiac failure, stroke, and renal failure.Reference Subramanyan, Joy and Balakrishnan11 The main cause of death is cardiac failure, as a result of hypertension or aortic regurgitation.Reference Subramanyan, Joy and Balakrishnan11, Reference Ishikawa and Maetani26
Table 2 Clinical classification of Takayasu’s disease as described by Ishikawa.Reference Ishikawa24

Serological investigations
The erythrocytic sedimentation rate is elevated in active disease in up to three-quarters of those in all age groups,Reference Kerr, Hallahan and Giordano7, Reference Ishikawa24 but it is a poor predictor of death and acute events. In one small study, albeit of only four patients, who had bypass surgery, and histologically proven active disease from arterial biopsy at the time of surgery, only one patient had an elevated erythrocytic sedimentation rate.Reference Kerr, Hallahan and Giordano7 A further study found that, despite multiple serological investigations, no test was reliably able to distinguish between healthy volunteers and patients with active Takayasu’s arteritis.Reference Hoffman and Ahmed27 Recently, work has focused on the role of inflammatory cytokines. Interleukin-6 and interleukin-18 are elevated in Takayasu’s disease. Interleukin-18 correlates well with disease activity and may prove a useful marker for monitoring treatment response.Reference Park, Lee, Park and Lee28
Radiological investigations
Chest radiograph
Changes in the appearance of the aorta manifest as abnormal calibre, along with aortic calcification in some. Undulation of the aortic margin can be seen, with alternating areas of stenosis and dilation, and also with skip areas of involvement. If segmental calcification is seen to outline an area of aortic narrowing, this is characteristic of Takayasu’s disease.Reference Berkmen and Lande29
Prominent pulmonary arteries signify pulmonary hypertension. Involvement of the intrapulmonary arterial branches may result in areas of oligaemia. Oligaemic lungs on plain chest radiography correlate with pulmonary arterial disease in one-third.Reference Sharma, Rajani, Kamalakar, Kumar and Talwar30
Rib notching occurs in the presence of collateral arterial flow secondary to aortic or subclavian artery stenosis, but is unusual since it takes many years to develop.
Hilar lymphadenopathy has been described.Reference Berkmen and Lande29 There are, however, a few reports of the coexistence of sarcoidosis and large vessel vasculitis,Reference Kerr, Hallahan and Giordano7, Reference Weiler, Redtenbacher, Bancher, Fischer and Smolen31 both of which may be steroid responsive, and therefore if seen, a coexistent disease should be considered.
Ultrasound
Ultrasound demonstrates homogenous, intermediate echoic circumferential thickening of the involved vessels, which is quite different from that seen in ordinary atherosclerosis.Reference Schmidt, Nerenheim, Seipelt, Poehls and Gromnica-Ihle32 This finding, particularly in young women, is highly specific for Takayasu’s disease. In transverse section, the circumferentially thickened intima-media complex is termed the “macaroni sign”.Reference Maeda, Handa and Matsumoto33 Other findings include vascular dilation, occlusion, and acceleration of flow through regions of stenosis. A reduction in diameter of one-half is required to modify the triphasic Doppler signal in the peripheral arterial tree.Reference Sun, Yip, Jeng, Hwang and Lin34 Visualisation of the abdominal vasculature is difficult, but can be partially overcome with transoesophageal imaging of the thoracic aorta, or by intravascular ultrasound.Reference Gotway, Araoz and Macedo35 Transcranial Doppler may be used to assess the intracranial arteries. In one study, sensitivity and specificity for transcranial Doppler and magnetic resonance angiography were both greater than 95%.Reference Cantu, Pineda and Barinagarrementeria36 The main advantage of colour Doppler flow imaging over magnetic resonance angiography is its ability to visualise residual blood flow. Overestimation of stenosis by magnetic resonance angiography is a well-known phenomenon related to intravoxel dephasing resulting from turbulence of flow at the narrowed segment.
Angiography
Digital subtraction angiography, of the aorta and its branches, has traditionally been the method for definitive diagnostic assessment.Reference Johnston, Lock and Gompels37 Luminal changes range from smooth tapering stenoses to frank occlusion, and collateral vessels may be seen.Reference Park, Han, Kim, Oh, Park and Seo38 The coronary arteries can be evaluated at the same time as the aorta, and if a systemic vein is cannulated, then the pulmonary circulation can be visualised.Reference Yoshida, Akiba and Tamakawa39 Angiography is essential if percutaneous intervention is to be considered, for definitive sizing of balloon angioplasty and for deployment of stents. The technique, however, is invasive, involves use of iodinated contrast, and a substantial dose of radiation is given to the patient. It shows arterial mural thickness poorly, and luminal abnormalities are generally a late feature.Reference Gotway, Araoz and Macedo35 There are cases where arterial puncture would be difficult because of extensive stenosis or calcification. In one report, the frequency of ischemic complications resulting from angiography was found to be high because blood coagulation is increased in patients with Takayasu’s disease.Reference Numano40
Cross sectional imaging
Computed tomography and magnetic resonance imaging are important tools for investigating patients, because they can assess luminal and mural changes, as well as angiographic appearances. In the acute inflammatory stage, the wall becomes thickened, a feature not seen in normal adults (Figs 5 and 6).Reference Park, Chung, Im, Kim, Park and Han41 A decrease in mural thickness following treatment with steroids has also been demonstrated with both modalities.Reference Hayashi, Fukushima, Matsunaga and Hombo42–Reference Tanigawa, Eguchi and Kitamura43

Figure 5 Axial computerised tomogram after administration of contrast at the level of the origin of the arch vessels. Soft tissue thickening surrounding all branches can be seen. Note the normal luminal calibre at this stage.

Figure 6 Axial T1-weighted magnetic resonance image at the level of the origin of the arch vessels. Soft tissue thickening surrounding all branches can be seen. The lumen of the left common carotid is now narrowed however.
Following administration of contrast, there is both early and delayed enhancement. On computed tomography, early mural enhancement is heterogeneous, whereas delayed enhancement is extremely pronounced. An inner concentric ring of low attenuation may also be seen on both arterial phase and delayed imaging in between the opacified blood and outer wall of the aorta, which most likely represents the intima. On magnetic resonance imaging, patterns of aortic mural enhancement are variable.Reference Choe, Han, Koh, Kim, Do and Lee44 In the acute phase, the aortic wall and surrounding adventitia enhance more than the myocardium, suggesting active disease. Using an inversion recovery prepared gradient echo sequence to null the signal from blood and the arterial wall, it is possible to show delayed mural enhancement (Fig. 7). The mechanism of enhancement using this technique is unknown. Delayed gadolinium enhancement is also seen with myocardial necrosis, fibrosis, myocarditis, and in atherosclerotic plaques.Reference Desai, Stone, Foo, Hellmann, Lima and Bluemke45 Some authors have not found the presence or absence of gadolinium enhancement of the arterial wall to be a reliable tool for assessing disease activity.Reference Andrews, Al-Nahhas and Pennell46

Figure 7 Delayed gadolinium enhanced magnetic resonance image. Axial image of the aorta at the level of the diaphragm, showing persisting enhancement at 20 minutes.
In chronic disease the wall becomes calcified, best appreciated on computed tomography. This calcification may be intimal, or more commonly fullthickness, reflecting the trans-mural nature of the inflammatory process. In addition, the arterial wall in chronic disease does not enhance on early or delayed imaging with iodinated contrast media.Reference Park, Chung, Im, Kim, Park and Han41 Computed tomography also demonstrates parenchymal change within the lungs. Areas of low attenuation reflect arteritis and hypoperfusion,Reference Paul, Hernigou and Lefebvre15 while wedged shaped areas of high attenuation suggest pulmonary infarction.Reference Nakamura, Hayashi, Fukuoka, Sueoka and Nagasawa47
Cross sectional imaging is of particular importance in follow-up. New lesions can be demonstrated in patients with clinical remission (Figs 8 and 9). Therapeutic effectiveness of medical and surgical intervention can be assessed, including treatment with steroids, the patency of bypass grafts, and any restenosis after percutaneous transluminal angioplasty. Magnetic resonance imaging has several advantages over computed tomography. Paramagnetic contrast media is not as nephrotoxic, and allergic reactions even rarer. There is no ionizing radiation, and hence it can be used safely for long term follow up. Soft-tissue contrast resolution is better, with improved visualisation of mural edema, and cine magnetic resonance imaging can be used to evaluate vascular and valvar flow, along with ventricular function.

Figure 8 Volume rendered magnetic resonance image subsequent to administration of gadolinium showing the aortic arch and its branches, and demonstrating mild tapering of the distal carotid arteries.

Figure 9 Volume rendered magnetic resonance image after administration of gadolinium of the aortic arch and branches, demonstrating a severe long segment stenosis of the left common carotid artery involving its origin, and mild narrowing of the other arch vessels. Figure 9 was obtained in the same patient 5 months after Figure 8, and shows progression of disease despite normal inflammatory markers, and no symptoms.
Positron emission tomography
Recently, [18F] fluorodeoxyglucose positron emission tomography has been investigated as a tool for diagnosing and monitoring disease activity in large vessel vasculitides. [18F] fluorodeoxyglucose identifies areas of high glucose metabolic activity.Reference Andrews, Al-Nahhas and Pennell46 Several reports have shown the value of this technique in the diagnosis of large vessel arteritis. In one study, 28 positron emission tomography scans were performed on 18 patients suspected of having Takayasu’s disease. All patients were evaluated with a full clinical and laboratory assessment, cross-sectional imaging and angiography, and all but 2 satisfied the criterions of the American College of Rheumatologists for Takayasu’s disease. In another study, the technique achieved a sensitivity of 92%, a specificity of 100%, and negative and positive predictive values of 85% and 100% respectively, in the initial assessment of active vasculitis in Takayasu’s disease.Reference Webb, Chambers and AL-Nahhas48 The signal is not always strong enough to diagnose inflammation, and sensitivity can be improved by co-registering with enhanced computed tomography.Reference Kobayashi, Ishii and Oda49 Accumulation of [18F] fluorodeoxyglucose has been shown to correlate with levels of activity of the disease, suggesting that it may be a potential tool for estimating the extent of disease and its response to medical treatment.Reference Andrews, Al-Nahhas and Pennell46, Reference Kobayashi, Ishii and Oda49
[18F] fluorodeoxyglucose has also been shown to accumulate in atherosclerotic plaques.Reference Davenport, Kirkpatrick, Arch, Pickard and Weissberg50 It should be noted that, as with other chronic inflammatory diseases such as systemic lupus erythematosus and rheumatoid arthritis, one-quarter of patients with Takayasu’s disease have an increased incidence of atherosclerosis.Reference Seyahi, Ugurlu and Cumali51 Interpretation of positive results, therefore, should be undertaken cautiously, and used in conjunction with clinical findings. Positron emission tomography with or without co-registration with computed tomography carries a high radiation dose. There is also limited availability, making it unsuitable for long-term follow up.
Treatment
Medical
Treatment is divided into those therapies designed to induce remission, and those that manage the complications of Takayasu’s disease. Glucocorticoid therapy is often the first line treatment. In one North American series,Reference Kerr, Hallahan and Giordano7 glucocorticoid therapy alone achieved initial remission in over half the patients, with children and adults achieving comparable levels of remission, but with shorter times to remission in children. Second-line agents, including cyclophosphamide, azathioprine, and methotrexate, can be added if the patient is unresponsive to glucocorticoids alone. This produces remission in up to four-fifths of patients, children as wells as adults.Reference Kerr, Hallahan and Giordano7, Reference Hoffman, Leavitt, Kerr, Rottem, Sneller and Fauci52 Increasingly, second line agents are being added early as steroid-sparing drugs, as opposed to waiting for relapse when steroids are weaned.Reference Ozen, Duzova and Bakkaloglu53 Minocycline combined with prednisolone has also been used with success in patients with active disease.Reference Matsuyama, Sakai, Ishigami, Hiraoka and Yamashita54 Use of anti-tumour necrosis factor therapy, with etanercept or infliximab, also produced an improvement in all but 1 patient with active relapsed disease over an age range of 17 to 48 years, and sustained remission in two-thirds of patients, who were able to discontinue glucocorticoid therapy.Reference Hoffman, Merkel, Brasington, Lenschow and Liang55
Hypertension can be difficult to treat, and is exacerbated by glucocorticoid therapy. The use of inhibitors of angiotensin converting enzyme needs particular care because of the high frequency of associated renal arterial stenosis.Reference Johnston, Lock and Gompels37 Thrombosis is a further problem, with patients often requiring antiplatelet therapy and anticoagulation. Prophylaxis against Pneumocystis carinii pneumonia may be required with immunosuppressant therapy. In one study, one-fifth of patients had strongly positive skin tests for tuberculosis, and had been started on anti-tubercular therapy.Reference Subramanyan, Joy and Balakrishnan11
Endovascular and surgical
For patients who require interventional revascularisation, both endovascular and surgical procedures can be performed safely, with low morbidity and mortality. The best long-term outcomes however are achieved with conventional bypass grafts.Reference Liang and Hoffman56 Interventional procedures are indicated for hypertension associated with critical renovascular stenosis, ischemia when extremely limiting activities of daily living, clinical features of cerebrovascular ischaemia or critical stenosis of at least three cerebral vessels, moderate aortic regurgitation, cardiac ischaemia with proven coronary arterial stenosis,Reference Kerr, Hallahan and Giordano7 and aneurysmal dilation of the aorta.
Bypass surgery, and the use of interposition grafts, with the exception of selected pulmonary arterial reconstructions, is the preferred technique for arterial reconstruction. This is because of disappointing early results with patch angioplasty and endarterectomy in areas of inflammation and transmural fibrosis. For bypass surgery, areas of the arterial tree that are unaffected by disease are the preferred anastomotic sites. Autologous grafts are more successful than synthetic grafts. Long-term rates of patency are good for both, but can be compromised by poor flow, if extensive collaterals divert large volumes of blood.Reference Weaver, Yellin and Campen57
Whenever possible, surgery should be done when the disease is quiescent, since there is an increased risk of failure for surgical procedures undertaken during active disease. Surgical bypass is the preferred treatment of longer segment stenoses and occlusions. Complications include restenosis, thrombosis, haemorrhage, infection,Reference Kerr, Hallahan and Giordano7 and anastomotic aneurysms.Reference Weaver, Yellin and Campen57
Percutaneous transluminal angioplasty with or without placement of stents has been described in the aorta, brachiocephalic, carotid, subclavian, coeliac trunk, mesenteric, iliac and femoral arteries. The success rate of angioplasty in total occlusion is comparatively low. Short-segment occlusions may be initially opened by angioplasty,Reference Tyagi, Verma, Gambhir, Kaul, Saha and Arora58 and can be successfully reattempted with recurrent stenosis. Endovascular stenting is performed following arterial dissection, and suboptimal results after angioplasty.
Initial success rates of angioplasty are high. In contrast to the results in treating atherosclerosis, nonetheless, a number of series report a high proportion of vessels restenosing.Reference Fava, Foradori and Garcia59, Reference Liang, Tan-Ong and Hoffman60 Other authors,Reference Sharma, Saxena, Talwar, Kaul, Mehta and Rajani61 in contrast, are more optimistic about the long-term efficacy of angioplasty. Coronary arterial stenting deserves particular attention, because of the seriousness, in terms of major adverse cardiac events, of occlusion of stents. There have only been a few reports regarding angioplasty and stenting of the coronary arteries resulting from Takayasu’s disease. The most likely reason is that lesions are usually sited at the orifices of the arteries, such as the main stem of the left coronary artery, which in the past have often been considered unsuitable for percutaneous treatment.Reference Sakai, Oyama, Kishimoto, Takahashi, Urasawa and Tsutsui62 There are promising early results, nonetheless, in the use of sirolimus-eluting stents. Sirolimus has immunosuppressive properties, which may have a beneficial synergistic effect with its anti-proliferative action on vascular smooth muscle cells in the prevention of occlusion after stenting the coronary arteries in Takayasu’s disease.Reference Furukawa, Tamura and Toma63 The surgical treatment of aortic regurgitation caused by Takayasu’s disease is difficult because of the need to manipulate friable tissue. Complications, such as valvar detachment after replacement of the aortic valve, or anastomotic aneurysm after composite graft repair, may still occur as a result of fragility of the aortic wall or the hingelines of the leaflets. There is also further concern about late dilation of the remaining ascending aorta in the long-term follow up.Reference Matsuura, Ogino and Kobayashi64
Conclusions
The investigation and management of Takayasu’s disease can prove difficult. The initial symptoms and signs are non-specific, and a high index of suspicion is needed if the diagnosis is to be made. The disease is associated with a high incidence of morbidity, and a significant risk of premature death. The presence of one or more severe single complications, including retinopathy; secondary hypertension; aortic regurgitation, and aortic or arterial aneurysms, are useful in predicting outcome. Serological tests have proved unreliable in distinguishing active from quiescent disease. Recent work suggests that monitoring inflammatory cytokines such as interleukin-18 may prove useful in the future.
The clinical challenge is to diagnose the disease early during active inflammation. Subtle signs may be apparent on plain radiography, but computed tomography is likely to be the most effective, widely available, tool for patients. Angiography has traditionally been the gold standard, but only provides information relating to luminal change. Magnetic resonance imaging is preferred for long-term follow up, as it visualises the vessel lumen, shows mural thickening, and enhancement, and can therefore provide information relating to activity of the disease and unrecognised complications. Positron emission tomography co-registered with computed tomography can also demonstrate active inflammation, but the availability is currently limited and requires a high radiation dose.
Medical treatment involves high dose steroids, with immunosuppressants. Newer agents, including anti-tumour necrosis factor drugs, have been used with some success. Surgical and endovascular procedures provide symptomatic relief from ischaemic complications, but suffer from late vascular occlusion. Future treatment may involve targeting both inflammation and myointimal hyperplasia associated with the disease in the form of medical therapy and drug-eluting endovascular stents.
Acknowledgements
We thank Athimalaipet Ramanan, consultant paediatric rheumatologist at Bristol Royal Hospital for Children, for his comments regarding medical therapy in Takayasu’s disease.














