Mortality has declined for infants with CHD who undergo palliative or corrective cardiothoracic surgery.Reference Latal 1 In addition to survival until hospital discharge, measurable outcome objectives for this population now consist of minimising perioperative morbidities, reducing hospital length of stay and cost, and improving neurodevelopmental function.Reference Jacobs, O’Brien and Jacobs 2 Early and consistent nutrition support after surgery may help to improve patient outcomes.Reference Kaufman and Barrett 3 Unfortunately, neonates and infants with CHD often struggle to achieve adequate enteral nutrition in general and with oral feeding specifically. These patients, particularly those with single-ventricle physiology, have an increased incidence of necrotising enterocolitis.Reference McElhinney, Hedrick and Bush 4
Necrotising enterocolitis is an intestinal pathology that was first described in preterm infants. The pathophysiology involves compromise of the intestinal mucosal barrier with subsequent invasion of pathogenic bacteria and resultant injury to the intestinal wall. The process can progress to bowel ischaemia, bowel perforation, sepsis, and death.Reference McElhinney, Hedrick and Bush 4 The severity of necrotising enterocolitis is graded by the modified Bell’s criteria (Supplementary Fig S1) and broadly categorises the pathology into suspected, confirmed, or advanced.Reference Kliegman and Walsh 5 However, the clinical presentation of necrotising enterocolitis may be variable and at times subtle. It may manifest along a continuum of irritability, feeding intolerance, abdominal distension, hematochezia, systemic inflammatory response syndrome, to haemodynamic collapse.Reference Giannone, Luce, Nankervis, Hoffman and Wold 6
The susceptibility of infants with CHD to necrotising enterocolitis is complex and multifactorial; some have theorised that it may have a different pathophysiology than necrotising enterocolitis in the preterm population.Reference Pickard, Feinstein, Popat, Huang and Dutta 7 Many factors may contribute to this pathophysiology. Mesenteric blood flow may be impaired in the setting of ductal-dependent systemic or pulmonary blood flow,Reference Dewitt, Charpie, Donohue, Yu and Owens 8 large left-to-right shunts, or cardiac rhythm disturbances.Reference Giannone, Luce, Nankervis, Hoffman and Wold 6 Additionally, blood flow and oxygen delivery to the intestines may be impaired owing to cyanosis, thrombotic or infectious events, or merely the presence of indwelling umbilical catheters.Reference Giannone, Luce, Nankervis, Hoffman and Wold 6 , Reference Motta, Scott and Mahony 9
Our goal was to quantify the incidence of suspected, confirmed, and advanced necrotising enterocolitis in infants <6 months old who underwent cardiac surgery. Our hypothesis was that, despite our low institutional rate of confirmed or advanced necrotising enterocolitis, suspected necrotising enterocolitis is common and it leads to interruptions of feeds and longer length of stays. We also sought to document the treatment response to all stages of necrotising enterocolitis and assess variability in practice patterns.
Materials and methods
We performed a retrospective chart review of children less than or equal to 6 months of age who underwent cardiac surgery from 1 January, 2012 through 31 December, 2013. Through review of the electronic health record, we identified all patients who had feeding difficulties or clinical exam findings suspicious for necrotising enterocolitis that led the care team to interrupt the enteral feeding regimen or obtain abdominal radiographs at any time point during their surgical admission. We then categorised the episodes into suspected (Grade IA and IB), confirmed (Grade IIA or IIB), or advanced (Grade IIIA or IIIB) necrotising enterocolitis using the modified Bell’s criteria. If the patient experienced a recurrence of necrotising enterocolitis during their hospitalisation, only their first episode during that hospitalisation was included in our analysis. If the patient underwent more than one hospitalisation, demographic and patient characteristics, excluding those associated with bowel pathology, were used once in the analysis.
Patients were excluded if they received pre-operative or post-operative care outside the cardiac ICU or our cardiology inpatient floor unit, had surgery for a non-cardiac diagnosis, had extracorporeal membrane oxygenation as the primary indication for surgical intervention, or had a gastrointestinal diagnosis that precluded provision of enteral feeds (such as Hirschsprung’s disease or imperforate anus). Patients with prematurity undergoing ligation of a patent arterial duct were excluded as their pre-operative and post-operative care was in the neonatal ICU. Feeding interruptions for a planned procedure or intervention were not included.
Both the cardiac intensive care and the cardiac inpatient care teams use a standardised feeding protocol (Supplementary Fig S2) and a nutrition flow sheet.Reference Kaufman, Vichayavilas and Rannie 10 The protocol dictates that enteral feeds be initiated in the well-perfused patient with a benign abdomen. Exclusion criteria for feeds include abdominal girth changes >10%, haemoccult positive stool, emesis, and bilious residuals. For the extubated, stable neonate, bolus feeds are started at 20 ml/kg/day divided into eight feeds, given over 1 hour. Feeds are advanced by 20–30 ml/kg/day. The extubated, stable infant with prior feeding experience is initiated on 10–60 ml/feed, given over 30–60 minutes and advanced by 20–40 ml with each feed. Continuous trophic feeds are started for neonates who were nil per os since birth or infants at risk for decreased gastrointestinal perfusion. Trophic feeds are initiated at 0.5 ml/kg/hour for 24 hours before advancing by 0.5 ml/kg/hour every 12 hours. For patients on mechanical ventilation, with previous feeding intolerance or otherwise determined to be too unstable for bolus feeds, continuous transpyloric feeds are initiated at trophic rates and similarly advanced.
For the purpose of data collection, patients who were ⩽14 days of age at the time of their surgical intervention had their birth weight recorded for weight at the time of surgery. For those patients who were >14 days of age at the time of surgery, their weight on the day of surgery was recorded. Gestational age was defined as weeks of gestation at the time of birth. Three patients had a gestational age recorded as “term”. For those patients, a gestational age of 39 weeks was used.
Each episode of necrotising enterocolitis prompted a review of the patient’s hospital admission including the duration of therapeutic nil per os time, antibiotic regimen and duration, surgical consultative practices, presence of progression of Bell’s stage after diagnosis, and need for abdominal surgical intervention. As neonates made up a large portion of this population, subgroup analyses of neonates, single-ventricle patients, and single-ventricle neonates were performed.
Categorical variables were compared using a χ2 test with Bonferroni correction. Continuous variables were compared using Kruskal–Wallis test with post hoc analysis using pairwise matching and Bonferroni correction. Median and interquartile ranges are reported. A p-value of <0.017 was considered significant for pairwise comparisons of categorical variables and <0.008 for continuous variables.
Results
A total of 293 patients ⩽6 months of age underwent cardiac surgery during the calendar years of 2012 and 2013 (Fig 1). A total of 251 patients were included in the analysis, representing 265 hospital admissions. In all, 42 patients were not included in the analysis owing to meeting the exclusion criteria (Supplementary Table S1). Patient demographics are listed in Table 1.

Figure 1 Overview of patients included in the analysis. NEC=necrotising enterocolitis; NICU=neonatal ICU.
Table 1 Characteristics and outcome data of children ⩽6 months of age undergoing cardiac surgery with and without necrotising enterocolitis (NEC)

The p-value in bold is for the χ2 comparison
For pairwise comparisons: ap-value when no NEC and suspected NEC cohorts are compared; bp-value when No NEC and confirmed NEC cohorts are compared; cp-value when suspected NEC and confirmed NEC cohorts are compared; p-value <0.017 is considered significant for categorical variables and p-value <0.008 for continuous variables
A total of 34 patients had 35 admissions during which they met criteria for suspected, confirmed, or advanced necrotising enterocolitis. In all, 19 of these episodes (n=18 patients; one patient had two separate admissions) were suspected necrotising enterocolitis and 16 episodes (n=16 patients and 16 hospitalisations) were confirmed or advanced necrotising enterocolitis. The overall incidence of suspected necrotising enterocolitis was 7.2% (Bell’s IA episodes, n=11; IB, n=8). The overall incidence of confirmed (IIA episodes, n=13; IIB, n=2) or advanced necrotising enterocolitis (IIIA episodes, n=1) was 6%. Both suspected and confirmed necrotising enterocolitis occurred more frequently in patients with younger age, lower weight, and single-ventricle physiology. Half (50%) of the patients who had suspected or confirmed necrotising enterocolitis had single-ventricle physiology. Length of hospitalisation was significantly longer in patients with suspected necrotising enterocolitis (p=0.001) and confirmed or advanced necrotising enterocolitis (p<0.001) when compared with those with no necrotising enterocolitis history. We examined the frequency in which patients with necrotising enterocolitis underwent surgical gastrostomy tube placement during their hospitalisation. Patients with either suspected or confirmed necrotising enterocolitis were more likely to undergo gastrostomy tube placement before hospital discharge (Table 1).
Progression of Bell’s staging was noted in three patients and recurrence of symptoms/signs of necrotising enterocolitis occurred in four patients (one with suspected and three with confirmed necrotising enterocolitis) during their surgical hospitalisation. For those patients with recurrence of necrotising enterocolitis, three of these patients had single-ventricle physiology and the fourth had an immunodeficiency. One patient’s recurrence fit classification for fulminant necrotising enterocolitis. Of note, one of the patients with suspected necrotising enterocolitis died secondary to worsening cardiac function in the setting of prematurity and congenital complete heart block requiring immediate postnatal pacemaker placement. This patient also had heterotaxy with left atrial isomerism.
Patients were fed according to the standardised feeding protocol as described in the Materials and methods section. The majority of patients with bowel pathology were receiving feeds via nasogastric tube or both nasogastric and oral at the time of necrotising enterocolitis onset (Table 2).
Table 2 Characteristics of patients who were diagnosed with suspected, confirmed or advanced necrotising enterocolitis (NEC).

ECMO=extracorporeal membrane oxygenation; IQR=interquartile range; NPO=nil per os; VAD=ventricular assist device
* If applicable
We examined the timing of onset of suspected or confirmed necrotising enterocolitis in relation to the patient’s cardiac operation. The diagnosis-suspected necrotising enterocolitis occurred significantly earlier in a patient’s post-operative course, whereas confirmed or advanced necrotising enterocolitis tended to be sporadic throughout the patient’s hospitalisation (Table 2 and Fig 2). Two patients were diagnosed with necrotising enterocolitis – one suspected and one confirmed –before their cardiac surgical intervention. Both patients were nil per os at the time. One patient was admitted in critical condition with a postnatal diagnosis of coarctation of the aorta and the other patient had single-ventricle physiology and underwent a lung biopsy before single-ventricle palliation. No other patients in our cohort who received enteral feeds preoperatively, including those with single ventricle physiology, had abdominal pathology during the pre-operative period.

Figure 2 Timing of NEC following Cardiac Surgery. NEC=necrotising enterocolitis; OR = operating room.
Additionally, we sought to document the clinical team’s treatment response to bowel pathology (Table 2). For all patients with necrotising enterocolitis, the median duration of nil per os time was 2 days. Of the patients with suspected necrotising enterocolitis, the median nil per os time was <24 hours. Of the patients with confirmed or advanced necrotising enterocolitis, the median nil per os time was 5 days. The majority (68%) of necrotising enterocolitis episodes of any classification were treated with antibiotics. The antibiotic regimens chosen varied widely – there were 13 different antibiotic regimens used in a total of 23 necrotising enterocolitis episodes.
A subgroup analysis of neonates was also performed (Table 3). There were no significant differences in patient characteristics between neonates with suspected, confirmed, or no necrotising enterocolitis. Neonates with confirmed or advanced necrotising enterocolitis remained hospitalised longer than neonates without (50 versus 18 days, p<0.0001).
Table 3 Characteristics of neonates with and without necrotising enterocolitis (NEC).

Bolded p-value is for χ2 comparison
For pairwise comparisons: ap-value when no NEC and suspected NEC cohorts are compared; bp-value when no NEC and confirmed NEC cohorts are compared; cp-value when suspected NEC and confirmed NEC cohorts are compared; p-value <0.017 is considered significant for categorical variables and p-value <0.008 is considered significant for continuous variables
Analyses of length of stay for single-ventricle patients of all ages and of single-ventricle neonates were performed (Table 4). Among single-ventricle patients of all ages, those who had confirmed or advanced necrotising enterocolitis had longer length of stay than those without necrotising enterocolitis. However, there was no significant difference in length of hospital stay in single-ventricle patients of all ages with suspected necrotising enterocolitis compared with those without necrotising enterocolitis. Among single-ventricle neonates, those with confirmed or advanced necrotising enterocolitis had significantly longer length of stay compared with those without necrotising enterocolitis.
Table 4 Length of stay in single-ventricle patients with and without necrotising enterocolitis (NEC).

The bolded p-value represents the Kruskal–Wallis ANOVA comparison
For pairwise comparisons: ap-value when no NEC and suspected NEC cohorts are compared; bp-value when no NEC and confirmed NEC cohorts are compared; cp-value when suspected NEC and confirmed NEC cohorts are compared; p-value <0.008 is considered significant for continuous variables
Discussion
In this cohort of neonates and infants with CHD, we found that suspected necrotising enterocolitis (Bell’s classification 1A and 1B is common, occurring slightly more frequently than confirmed necrotising enterocolitis, and is associated with increased length of stay. Although the causal relationship between suspected necrotising enterocolitis and longer length of hospitalisation cannot be definitively determined with a retrospective study of this nature, we speculate that the treatment team approaches advances to the patient’s care with more caution after a single episode of suspected necrotising enterocolitis. In addition, care of infants with suspected necrotising enterocolitis was widely variable in terms of antibiotic regimen chosen, length of antibiotic treatment, and therapeutic nil per os times. All these variabilities may adversely contribute to length of hospital stay. Suspected necrotising enterocolitis, although less dramatic and debilitating than confirmed or advanced necrotising enterocolitis, warrants continued attention – in terms of prevention, early recognition, and standardisation of treatment.
Previous published reports of necrotising enterocolitis in comparable populations demonstrated an incidence of 3.3–9%.Reference McElhinney, Hedrick and Bush 4 , Reference Scahill, Graham, Atz, Bradley, Kavarana and Zyblewski 11 , Reference Leung, Chau and Hui 12 These previous studies were largely focused on confirmed or advanced disease. We speculate that they underestimate the incidence of suspected necrotising enterocolitis. Our combined incidence of any classification of necrotising enterocolitis was higher – 13%. However, the incidence of confirmed or advanced necrotising enterocolitis in our cohort was similar at 6%.Reference McElhinney, Hedrick and Bush 4 , Reference Scahill, Graham, Atz, Bradley, Kavarana and Zyblewski 11 , Reference Leung, Chau and Hui 12 Similar to prior studies, we found that single-ventricle physiology, lower weight, and younger age were risk factors for both suspected and confirmed necrotising enterocolitis.Reference Giannone, Luce, Nankervis, Hoffman and Wold 6 Among our neonatal single-ventricle patients, the incidence of confirmed or advanced necrotising enterocolitis was 14%, similar to the 17–18% reported in the literature. 13 , Reference del Castillo, McCulley and Khemani 14 Notably, 19% of our single-ventricle population was identified as having suspected necrotising enterocolitis, which underscores the widespread fear of necrotising enterocolitis in this population.
The timing of necrotising enterocolitis events was notably different between suspected versus confirmed or advanced necrotising enterocolitis. Suspected necrotising enterocolitis occurred earlier in the patients’ post-operative course and coincided when most patients are initiating feeds for the first time post-operatively. Although a retrospective study of this nature cannot assign causation, we speculate that many of the suspected necrotising enterocolitis episodes, particularly those that were categorised as Bell’s 1 A, may in fact represent feeding intolerance, rather than true necrotising enterocolitis pathology.
The patients who experienced a recurrence of necrotising enterocolitis notably had confirmed necrotising enterocolitis with their first occurrence and risk factors for a more severe course, such as single-ventricle physiology and immunodeficiency.
Previous studies have examined the use of graduated feeding protocols and near infrared spectroscopy monitoring in the prevention of necrotising enterocolitis.Reference Kaufman, Vichayavilas and Rannie 10 Our institution has enacted an enteral feeding algorithm as detailed in the Methods section. A branch of this algorithm assists with a conservative advance of feeds for high-risk infants. At the time of this review, an audit revealed an institution-wide compliance rate of 70% of infants completing the algorithm. Overall, our data support the safety of this feeding protocol in the pre-operative patient, as no infants developed necrotising enterocolitis preoperatively while receiving enteral feeds. In general, our institution’s strategy is to allow oral feeds preoperatively, even in the setting of ductal-dependent pulmonary or systemic blood flow, unless the patient is deemed too unstable as detailed in the attached feeding protocol.
Although the feeding protocol has been helpful in reducing practice variability with provision and advancement of enteral feeds, it did not eliminate the variability in treatment of suspected and confirmed necrotising enterocolitis. Variability identified in treatment for necrotising enterocolitis in our cohort included the duration of nil per os time, antibiotic regimens, and consultation of surgical services. Despite variability of practice, progression of suspected necrotising enterocolitis to more serious necrotising enterocolitis was rare. Nonetheless, we recognise the possible unwanted and dangerous effects this can have on care of these patients. Therefore, as outcome of this project, we have developed and instituted care guidelines for the diagnosis and treatment of necrotising enterocolitis in the cardiac population (Tables 5 and 6). This is an ongoing quality improvement initiative and a collaborative effort with cardiac intensive care, cardiac surgery, paediatric surgery, paediatric gastroenterology, pharmacy, and cardiac nursing teams. We will track utility of the guideline and adherence to the recommended diagnostic and therapeutic steps, while closely monitoring the frequency of necrotising enterocolitis progression or recurrence. We are hopeful that this will decrease the variability in care and minimising necrotising enterocolitis progression, as well as recurrence.
Table 5 Care guidelines for the standard risk cardiac patient with necrotising enterocolits (NEC).
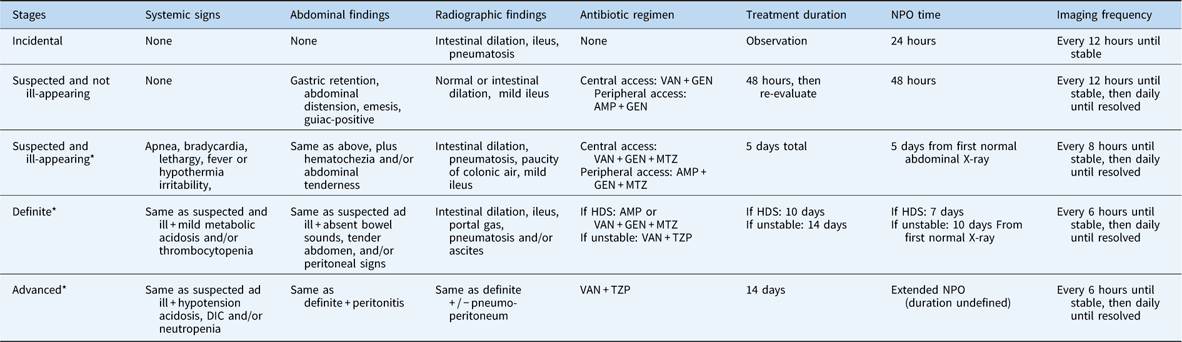
DIC = disseminated intravascular coagulation; GEN=gentamicin; HDS=haemodynamically stable; MTZ=metronidazole; NPO=nil per os; TZP=piperacillin-tazobactam; VAN=vancomycin
Standard risk defined by: right-sided obstructive lesion, mild congestive heart failure, heart transplant
Additional recommendations: blood cultures must be drawn before first dose of antibiotics. If blood cultures remain negative for organisms requiring vancomycin for 36 hours, ampicillin or piperacillin-tazobactam can be used. In cases of renal impairment, a third-generation cephalosporin can be used in place of gentamicin
* Surgical consultation recommended for all patients
Table 6 Care guidelines for the high risk cardiac patient with necrotising enterocolits (NEC).

DIC = disseminated intravascular coagulation; GEN=gentamicin; HDS=haemodynamically stable; MTZ=metronidazole; NPO=nil per os; TZP=piperacillin-tazobactam; VAN=vancomycin
High risk defined by: single-ventricle physiology, aortic arch obstruction, heterotaxy, significant shunt, significant congestive heart failure
Additional recommendations: blood cultures must be drawn before first dose of antibiotics. If blood cultures remain negative for organisms requiring vancomycin for 36 hours, ampicillin or piperacillin-tazobactam can be used. In cases of renal impairment, a third-generation cephalosporin can be used in place of gentamicin
* Surgical consultation recommended for all patients
Limitations to our study include those endemic to a single-centre retrospective chart review. In particular, the completeness of our data relies heavily on the diligence of the bedside nurse to document feeding interruptions and the medical provider to identify and document their clinical suspicion for necrotising enterocolitis. We sought to minimise this limitation by reviewing each chart individually, not by queries for billing codes. We are also unable to determine the causation of longer length of hospitalisation in these patients, only infer correlation. Additionally, the diagnosis and interpretation of radiographic pneumatosis is in itself variable and included multiple radiologists. This certainly could have contributed to difference in the interpretation of the radiographic findings and subsequent Bell’s classification. Finally, our subgroup analyses are limited by their small numbers.
In conclusion, suspected necrotising enterocolitis in infants with CHD is a significant contributor to length of hospitalisation after heart surgery. Suspected necrotising enterocolitis poses a unique challenge owing to the potential for uncertainty of diagnosis and recognition. Furthermore, variability of practice and treatment may extend hospital length of stay. Suspected necrotising enterocolitis warrants a broader conversation to establish safe, reasonable, and consistent management in the CHD population.
Acknowledgements
Jennifer Eshelman, PharmD BCPPS, is acknowledged.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees (Colorado Multiple Institutional Review Board).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951117002815










