Adult patients with single-ventricle pathology and limited pulmonary blood flow are a significant management challenge. Many of these patients have long-standing contraindications to venous palliation, and a proportion will be dependent on previously placed arterial shunts. In these cases, progressive cyanosis and exercise limitation are inevitably a major symptomatic burden. Transcatheter techniques can help improve pulmonary blood flow in these patients. Although described in children, there are very few studies in the literature of patients in this age group who present a unique technical and management challenge.Reference Dancea, Justino and Martucci 1
Materials and methods
In all, five patients after transcatheter intervention to arterial shunts were identified from our departmental database (Table 1). The mean age was 30.6 years (range 17–45). In all, four out of five patients were female. Written consent was obtained from all patients.
Table 1 Background and technical information.

ccTGA=congenitally corrected Transposition of the Great arteries; mBTS=modified Blalock–Taussig shunts; PAs=pulmonary arteries; VSD=ventricular septal defect
Patients’ cases are summarised in Table 1. All five patients had contraindications to further surgical venous palliation, either significantly raised pulmonary artery pressures or significant anatomical abnormalities of the pulmonary tree precluding a Glenn shunt.
All patients were uniformly severely symptomatic.
Shunt anatomy was as follows:
Two patients with central – aorta to pulmonary artery – shunts.
One with both a peripheral Gore-Tex – modified Blalock–Taussig – shunt and also a central connection.
One patient with both left and right modified Blalock–Taussig shunts.
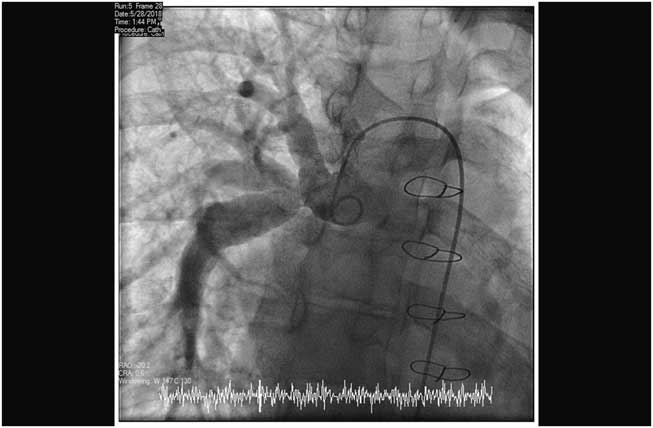
One patient with bilateral classical Blalock–Taussig shunts and one case with disconnected branch pulmonary arteries where a left classical Blalock–Taussig shunt supplied the left lung and a patent ductus arteriosis supplied the right lung (Table 1) (Figs 1–7).

Figure 1 Stenting classical right classical Blalock–Taussig shunt, positioning the stent.

Figure 2 Stenting classical right classical Blalock–Taussig shunt, pre-deployment angiogram.

Figure 3 Stenting classical right classical Blalock–Taussig shunt, post-deployment angiogram.

Figure 4 Stenting right modified Blalock–Taussig shunt, posteroanterior projection, pre-deployment angiogram.
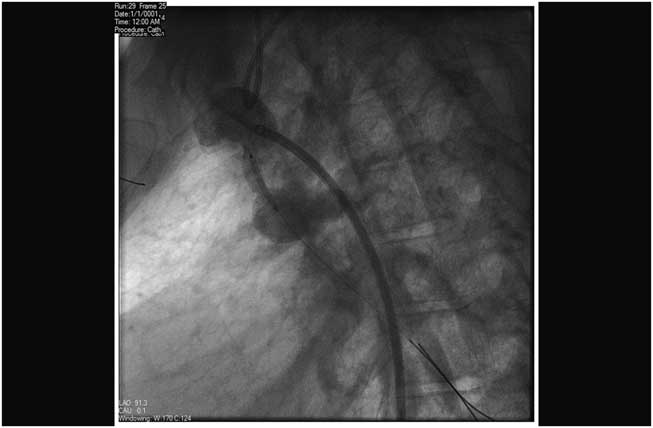
Figure 5 Stenting right modified Blalock–Taussig shunt, lateral projection, pre-deployment angiogram.

Figure 6 Stenting right modified Blalock–Taussig shunt, post-deployment angiogram.

Figure 7 Stenting right modified Blalock–Taussig shunt, post-deployment pulmonary angiogram.
Peripheral oxygen saturations, NYHA status, haemoglobin, and degree of atrio-ventricular valve regurgitation as assessed on transthoracic echo were compared before and 6–12 months after procedures to improve shunt flow.
Procedures are summarised in Table 1. In all five patients, pre-procedural cross-sectional imaging (MRI) was performed to allow for planning of the procedure and approach.
All patients continued with pre-existing anti-platelet therapy and received 100 iu/kg heparin intravenously during catheterisation, thereafter titrating to the activated clotting time >250 seconds. Following treatment, all were placed on single anti-platelet treatment with aspirin.
Results
There was a short-term improvement in oxygen saturations; the pre-procedure mean was 75.8 (SD 2.55)% (range 70–85%) and post-procedure mean was 83 (SD 2.52)% (range 78–87%), with a p value of 0.04. Haemoglobin level decreased from a pre-procedure mean of 22.06–20.28 g/L at 6 months post procedure (range 18.1–24.4 to 13–23.3 g/L), with a p value of 0.44. NYHA class decreased from a mean of 3.2–2.2 post procedure. Left atrial volume for four cases did not change (22.6–76.6 ml [mean 48.4 ml] to 29.6–72.9 ml [mean 52 ml], p value: 0.83). Only one case showed an increase in atrioventricular valve regurgitation from trivial to moderate, with the other cases showing no change (Table 2). Further follow-up of the cases (1–8 years, mean of 3.1) showed maintenance of symptomatic improvement in 4/5 cases; the remaining case developed pulmonary haemorrhage from systemic to pulmonary collaterals, resulting in a significant deterioration in symptoms independent of shunt status. Subsequent evidence of recurrent shunt/stent narrowing was noted in two patients on the follow-up based on symptoms and subsequent MRI imaging (Table 3).
Table 2 Medium term outcome post stenting arterial shunts.

Table 3 Longer-term follow-up post stenting arterial shunts.

BT, Blalock–Taussig; LBT, Left Blalock-Taussig
Discussion
Adults, palliated with arterial shunts, are a rare group of patients generally with anatomy and haemodynamics that preclude conventional surgical management. In the majority of cases, arterial shunts are constructed to provide palliation during early life while limiting exposure of the pulmonary bed to systemic pressures. As a result, the flow provided into the pulmonary circulation by an arterial shunt constructed in childhood will almost always be inadequate by adult life. In addition, arterial shunts have a tendency to thrombosis, growth-related distortion, and internal peel formation, which can further compromise flow.Reference Santoro, Bigazzi and Caianiello 2 By the time these patients reach adulthood, nearly all will suffer from severe progressive cyanosis, significant exercise limitation, and secondary polycythaemia. In many cases, these symptoms will be severely dehabilitating.
While further surgical palliation can occasionally be performed, it is considered to be very high risk, and rehabilitation of these shunts in the catheter laboratory using stents is a preferable option.Reference Vaughn, Moore, Mallula, Lamberti and El-Said 3
In our series, we were able to improve the luminal diameter of all of the shunts we encountered using a variety of stents delivered via standard arterial approaches. In all of the cases, there was an acute increase in saturation, a decrease in haemoglobin, and most importantly a marked rise in exercise tolerance, which has been maintained at a mean follow-up of 12 months post procedure.
Although the diameters of the implanting balloons used for stent delivery were only fractionally larger than the original diameter of the shunts (4–6 mm), flow through a tube is a function of the square of the diameter and so even small increases in shunt size would be expected to increase flow to the pulmonary arteries significantly. Using larger balloons would potentially risk acute pulmonary over-circulation and in any case the majority of shunts, particularly those constructed from Gore-Tex tubes, are resistant to significant over-dilation. At these sizes, there are a large number of pre-mounted stent systems available that have excellent tracking characteristics, making access to the shunt relatively straightforward, particularly when the procedure is planned using cross-sectional imaging to delineate the optimal approach.
In this series, the clinical improvements were maintained over the medium term (3 years median). All patients were maintained on a long-term single anti-platelet regimen. Whether these stented shunts are more or less prone to further distortion, neo-intimal ingrowth, or thrombus formation cannot be determined from this study. The study has also a limitation with the number of patients; this is likely to be because of the rarity of such presentation, and another limitation is the type of the stents used as we currently have no experience of drug-eluting stents in this environment.
In all, two of our patients developed restenosis of the shunt. This was suspected on the basis of the clinical and non-invasive investigations and confirmed either on MRI or angiogram. Both have anatomy suitable for restenting and are currently awaiting treatment.
Conclusions
Adult patients with arterial shunts carry a heavy symptomatic burden. Stenting of any intrashunt stenosis results in clinical improvement as demonstrated by improvements in saturations and clinical symptoms that are maintained in the medium term.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflicts of interest
None.