Published online by Cambridge University Press: 16 September 2005
Hunter in 1783,1 Hare in 1853,2 Peacock in 1871,3 and possibly Abercrombie in 1883,4 were amongst the first to describe the malformation now known as pulmonary atresia with intact ventricular septum. From the brief description provided by Abercrombie,4 it is unclear as to whether his patient had both tricuspid and pulmonary atresia, rather than pulmonary atresia with an intact ventricular septum, but at all events, his case was certainly an example of hypoplasia of the right heart. It was about eight decades ago, in 1926, that Grant5 first called attention to the pathological findings of a vascular connection between the right ventricle and a coronary artery in the heart from a 14-month-old female who died with this condition.5 Grant speculated that the high intracavitary pressure resulting from the atresia at the right ventricular outlet might be the cause of the vascular malformation. His suggestion was repeated nearly four decades later, in 1964, by Lauer et al.6 They published some of the earliest, and clearest, angiographic images of what they termed “intramyocardial sinusoids” in pulmonary atresia with an intact ventricular septum.6 While they demonstrated with clarity the angiographic features of these abnormal communications, they offered no suggestions for their potential role, either positive or negative. A decade later, in 1974, one of us (RMF) suggested, along with Harrington,7 that the abnormal vascular connections could contribute to myocardial ischaemia by compromising normal diastolic flow to the coronary arteries. The interest engendered in these ventriculo-coronary arterial connections has continued to the present time,8–22 as manifested by the many publications displaying the panorama of the angiocardiographic features, and many more discussing their influence on the outcomes of surgical intervention. Thus, with this increasing appreciation of the abnormal coronary circulation, and its influence on myocardial perfusion, algorithms were defined to neutralize as far as possible this morphological variable in patients undergoing surgery.23–33 Our review will summarize the history of these fascinating communications, charting the journey that took them from a once pathologic curiosity to a recognized risk factor in the surgical management of a select group of patients with pulmonary atresia and intact ventricular septum, and emphasizing the steps taken to neutralize their effect.
The malformation we will discuss has been described, over the years, in several ways. The terms used have included pulmonary atresia with normal aortic root, and pulmonary atresia with intact ventricular septum. The lesion can also be considered as hypoplasia of the right heart. Our preference is for pulmonary atresia with an intact ventricular septum.
The connections between the cavity of the right ventricle and coronary arterial bed have also been described in several ways.10, 11, 16, 34, 35 Although often designated as “sinusoids”, this is incorrect. We will use the terminology advocated by Gittenberger-de Groot et al.,36 who have written extensively on the topic. They characterize the ventriculo-coronary arterial connections as thick-walled and distended intertrabecular myocardial spaces, which connect with the subepicardial coronary arteries through a more-or-less distended capillary bed36 (Fig. 1).
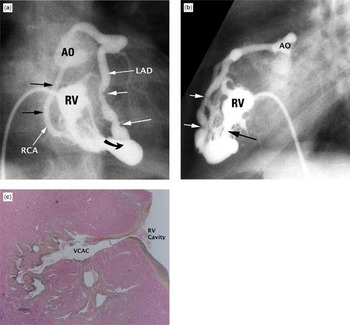
Figure 1. Ventriculo-coronary arterial connection in pulmonary atresia and intact ventricular septum. (a) Frontal right ventriculogram shows a hypoplastic right ventricle with attenuation of all three components of its cavity. Both the right (RCA) and the anterior descending coronary artery (LAD) clearly connect with the aortic root (AO). The right ventricle (RV) also connects via a ventriculo-coronary connection (curved arrow) with the coronary circulation. The white arrows show mild narrowing in the anterior descending coronary artery. The right coronary artery (black arrows) is mildly dilated. (b) The lateral projection of the injection showed in (a) shows ectasia and narrowing of the anterior descending coronary artery (white arrows). The connection between the right ventricle and the coronary circulation is marked by the black arrow. (c) A hematoxylin and eosin stain shows a ventriculo-coronary arterial connection (VCAC) between the cavity of the right ventricle (RV) and a coronary artery.
In almost all of the patients having pulmonary atresia with an intact ventricular septum the heart is normally positioned in the mediastinum, with usual atrial arrangement and concordant segmental connections, and with a left-sided aortic arch.36–39 A few patients with pulmonary atresia and an intact ventricular septum have discordant atrioventricular and ventriculoarterial connections, so-called double-discordance, or congenitally corrected transposition, and in some of these, the heart has been right-sided.21, 40–42 Typically, the pulmonary arteries are confluent, and are supplied by a left-sided arterial duct.36–39 Rarely, the pulmonary arteries can be non-confluent, with each artery supplied by its own arterial ducts, or else by an arterial duct, or a so-called fifth aortic arch, if this condition truly exists, or from direct aortopulmonary collateral arteries, an aortopulmonary fenestration, or some other combination.43–48 Confluent pulmonary arteries in combination with direct systemic-to-pulmonary collateral arteries can rarely be found, but this combination should raise the suspicion that initially there had been a ventricular septal defect that has closed spontaneously. When the pulmonary arteries are confluent then the pulmonary trunk is usually patent, but this is not always the case, and it can be thread-like (Fig. 2).36–39 We have reviewed elsewhere the detailed morphology of the heart itself when there is pulmonary atresia and the ventricular septum is intact.49 The association between conspicuous venous valves and maldevelopment of the right heart50, 51 is worthy of emphasis, nonetheless, this first being noted by Kauffman and Anderson in 1963.50

Figure 2. External view of the heart from a patient with pulmonary atresia and intact ventricular septum. The aorta (AO) is dilated, and the proximal pulmonary trunk (MPA) is represented by a fibrous cord (two small black arrows). The aortic arch is left-sided and the arterial duct (DA) supplies the confluent pulmonary arteries. The epicardial course of the left anterior descending coronary artery (LAD) demarcates a larger left ventricle (LV) from the smaller right ventricle (RV). The left anterior descending coronary artery originates from the dilated, ecstatic, and thickened right coronary artery (RCA).
Ventriculo-coronary arterial connections are virtually unknown when the malformation exists in the setting of a massively dilated and thin-walled right ventricle with a grossly abnormal and regurgitant tricuspid valve, the leaflets of the valve typically being dysplastic, displaced, or absent.52–54 These patients typically have a ratio between the right ventricular and the aortic or left ventricular systolic pressure that is less than one.53 Instead, the ventriculo-coronary arterial connections (Fig. 1) are typically found in those patients with gross underdevelopment of the cavity of the right ventricle, with marked stenosis of the tricuspid valve, and with hypertrophy of the ventricular walls sufficient to squeeze out the cavities of the apical and infundibular components, thus producing right ventricular hypertension8–21 (Fig. 3). It should be remembered, nonetheless, that ventriculo-coronary arterial connections have also been observed in patients with double outlet left ventricle with an intact ventricular septum, in aortic atresia with discordant ventriculoarterial connections and an intact ventricular septum, and when a common arterial trunk arises from the left ventricle in the setting of an intact ventricular septum (see Reference #55).

Figure 3. This specimen from a patient with pulmonary atresia and intact ventricular septum is dissected to demonstrate the massive hypertrophy of the ventricular septum and the right ventricular free-wall (RVFW). Note the difference in thicknesses between the right and left ventricles (LV). The subedocardial myocardium is pale, consistent with reduced perfusion and likely chronic ischaemic changes. The tricuspid valve (TV) is appreciably smaller than the mitral valves (MV). AO: aorta; RA: right atrium.
We have already made comment regarding Grant's description of ventriculo-coronary arterial connections.5 A decade later, in her now-famous Atlas of Congenital Cardiac Disease, Abbott56 collected 10 cases of pulmonary atresia with closed ventricular septum, culling 9 of these from the then extant literature, and suggested that the lesion was likely of an inflammatory origin, supporting her earlier published opinion.57 Bauer and Astbury58 have provided a detailed bibliography of the 1,000 cases analyzed in her Atlas.56 In this tabulation, references 509 through 518 address the 10 patients with pulmonary atresia and intact ventricular septum, and hence include the case report from Abbott herself, which is Citation #509.58 More than half a century later, Vigorita59 also speculated that the coronary arterial pathology seen in some of these patients might reflect a coronary endarteritis, a theme to which we will return. Abbott,56 however, did not mention the publication of Grant5 when compiling her list of cases. Indeed, as far as we can determine, none of these early papers, other than that of Grant, specifically mentioned the presence of the cameral-coronary connections, with some going so far as to doubt that such connections occurred. Any possible doubt was laid to rest, nonetheless, by the seminal paper of Wearn et al.60 Their report, published in 1933, begins: “The existence of direct vascular communications between the coronary arteries and the chambers of the heart has been claimed and denied”.60 Subsequent to their investigation, there could no longer be doubt about the reality of the connections.
Despite their account, the literature concerning pulmonary atresia in the setting of an intact ventricular septum remained modest until the late 1950s, and was accumulated mostly in the form of isolated case reports. As well as the list of such case reports compiled by Abbott,56 further lists are found in the compilation of Bauer and Astbury,58 and in the 1958 edition of the textbook of Keith et al.61 The connections, often described incorrectly as “myocardial sinusoids”, received no mention in several of the textbooks then devoted to paediatric cardiology, such as those of Taussig,62 Nadas,63 that of Keith et al. containing the list cited above,61 nor in either edition of the famous textbook on the diagnosis of congenital cardiac disease produced by those working at the Karolinska Institute in Sweden.64, 65 Furthermore, they still received no mention in the second edition of the textbook of Taussig,66 published in 1960. Indeed, in the first edition of the textbook of Nadas,63 there was no mention of the entity of pulmonary atresia with an intact ventricular septum itself. Even the second edition,67 published in 1963, devotes no more than a few pages to the malformation, commenting on its “malignant” nature. And still there is no mention of “myocardial sinusoids”! It is only in the third edition, published in 1972 with Fyler as co-editor,68 that “myocardial sinusoids” attract attention as one mechanism by which blood can exit from the blind right ventricle. According to most sources, the next significant mention of the connections between the right ventricle and the coronary arterial bed, following that of Grant,5 was the study of Williams et al., published in 1951.69 This work is important, since the authors demonstrated the histopathology of the involved coronary arteries, showing an eccentric lumen compromised by what they termed fibroelastic tissue.69
After this, the next significant step in the understanding of the communications came from the works published by Greenwold et al.70 in abstract form, and then by Davignon et al.71 as a full manuscript, with several of the same authors appearing in both publications. Their aim was to stratify the anatomic features of the malformation by means of clinicopathologic correlations. They identified a group with small right ventricles, contrasting these patients to those with either normal or enlarged right ventricles. In their second group, however, they did not include any of the patients with hugely dilated right ventricles and very abnormal and incompetent tricuspid valves. Figure 7 from their publication of 1956,70 a right ventriculogram profiled in frontal and lateral projections, clearly shows a hypoplastic right ventricle with opacification of what they termed “an anomalous coronary vessel and myocardial sinusoids”. They identified such communications in over two-thirds of their patients with small right ventricles.71 Somewhat surprisingly, this seminal investigation was not listed in the investigation of Lauer et al.,6 which is itself obviously of great importance. It was this investigation of Lauer et al.,6 as we have already emphasized, that provided the most beautiful angiograms to that date of the ventriculo-coronary arterial connections to be found in the setting of pulmonary atresia and intact ventricular septum, and correlated these findings with histopathology of the abnormal vessels. Indeed, we can date the subsequent waxing interest in these “peculiar” communications to the publication of the paper of Lauer et al.6
In the first paper discussing this lesion written by one of us (RMF) together with Harrington,7 we wrote “…it is possible that that the left ventricular ischaemia and dysfunction might be related to the intramyocardial-sinusoids-coronary artery communications in the patient with severe right ventricular hypertension”. We concluded this publication concerning what we then termed “myocardial sinusoids” by stating: “They serve as a passive conduit for the egress of blood from the blind right ventricle, and may hinder normal diastolic coronary perfusion and hence predispose to ischaemic myocardial dysfunction”.7 The evidence for these statements and conclusions was clearly derivative and inferential.
By this time, Esterly and Oppenheimer,72, 73 and others,74, 75 had fully documented the presence of abnormal coronary arterial pathology, and myocardial ischaemia or necrosis in patients, usually neonates, with congenital cardiac disease. Numerous amongst these patients were those having pulmonary atresia with an intact ventricular septum.72, 73 Fyfe et al.76 showed somewhat later that the myocardial ischaemia seen in the patients with pulmonary atresia and an intact ventricular septum was not invariably associated with an abnormal coronary circulation, a finding that we also substantiated. We were aware of the obstructive coronary arterial pathology that had been observed by others, citing at that time the experiences of Lauer et al.,6 and Williams et al.,69 but not the papers of Esterly and Oppenheimer,72, 73 although we were familiar with these works. In retrospect, considering the existence of the observations made by Williams et al.,69 and by Esterly and Oppenheimer,72, 73 it is perhaps surprising that, by the late 1960s, the abnormal coronary arterial pathology had not more directly been implicated by clinicians as being responsible for the adverse outcomes seen so frequently in these patients.
Thus, a number of electrocardiograms had been published from patients unequivocally exhibiting evidence of left ventricular ischaemia or infarction.68, 77, 78 Although written in the era prior to routine administration of prostanoids, the electrocardiographic changes, with the aid of the retrospectroscope, seem more conspicuous in patients with pulmonary atresia and intact ventricular septum than in similarly hypoxic neonates with tetralogy of Fallot, tetralogy with pulmonary atresia, or neonates with critical pulmonary stenosis. This, however, was a purely clinical and subjective observation, and no rigorous statistical analysis was performed.
Furthermore, we had also noted that, during attempted relief of the obstructed outflow tract in those patients with significant ventriculo-coronary arterial connections, whether by closed or open techniques, many of the patients developed important abnormalities of the ST-T segment coincident with ventricular decompression, followed shortly by intraoperative evidence of left ventricular ischaemia, severe left ventricular dysfunction, progressive systemic hypotension, a widening QRS complex, and an unresponsive bradycardia or chaotic disturbance of ventricular rhythm culminating in death of the patient.79 This sequence of events was also observed in babies palliated by construction of a systemic-to-pulmonary arterial anastomosis, but less so than in babies in whom the right ventricle was decompressed. These observations led us to believe that an untoward event involving the coronary arteries had taken place when right ventricular decompression was achieved. This observation was also seen somewhat later in our early experience with thromboexclusion of the right ventricle,80 a manoeuvre initially advocated and carried out by Waldman et al.81, 82
We also surmised that, since rapid clinical deterioration occurred following right ventricular decompression in some of these patients, myocardial perfusion might depend on retrograde flow from the hypertensive right ventricle to the coronary arterial bed. From these clinical and autopsy findings, we, and others, developed independently and almost simultaneously the concept of a right ventricular-dependent coronary arterial circulation.8, 13, 14, 18–22 These clinical and morphological observations were extended by publications from Toronto and elsewhere,9–12, 14–22, 39, 55, 83 being followed by other numerous publications characterizing the pathologic findings of the myocardial ischaemia and adverse coronary arterial pathology.14, 15, 18–24, 32, 39, 45, 49, 55, 80, 83
For the most part, the connections are found in the hearts from patients with severe right ventricular hypertension, tricuspid valvar stenosis or hypoplasia, and important right ventricular hypoplasia9, 12–22, 28, 33, 35, 49, 55, 71 (Figs 2 and 3). Exceptions have been documented, with important coronary arterial pathology observed in the occasional patient with a normally sized but hypertensive right ventricle.84 There tends, nonetheless, to be a mutually exclusive relationship between the presence of the connections and the absence of ventricular endocardial fibroelastosis.85–87 Thus, in those hearts with ventriculo-coronary arterial connections, right ventricular endocardial fibroelastosis, if present at all, tends to be mild, and patchy. If right ventricular endocardial fibroelastosis is dense and diffuse, it is most unlikely that significant ventriculo-coronary arterial connections will be present.85–87 Furthermore, it is likely that, in the hearts with the connections, mural hypertrophy will be severe, attenuating or obliterating the apical trabecular zone.38, 49 The infundibular component of the right ventricle may also be attenuated or absent, a finding we demonstrated more than three decades ago using the double-catheter technique88 (Fig. 4). Pawade et al.89, 90 subsequently used the absence of a patent infundibulum to guide surgical intervention, likely based on the observation of the normal right ventricle as a tripartite structure.91, 92 The corollary is that the right ventricle in the patient with pulmonary atresia and intact ventricular septum that lacks any patent infundibular component is the most disadvantaged, and the most likely to be associated with ventriculo-coronary arterial connections.

Figure 4. The lateral right ventriculogram (RV), using a double-catheter technique, reveals absence of the infundibulum of the right ventricle. MPT: pulmonary trunk.
In terms of their origin, epicardial distribution, and histology, the coronary arteries may be completely normal in the setting of pulmonary atresia and intact ventricular septum. Indeed, this is the situation in the majority of patients.49 Even amongst those patients with abnormal coronary arteries (Figs 5 and 6), there is a great deal of variability in the severity of the changes.7–22, 27, 28, 30–33, 49, 55, 83, 93–95 Some patients will have only small fistulous communications, which may involute completely after satisfactory decompression of the right ventricle. In other patients, the involved coronary artery may show important stenosis at one or more sites, or even complete interruption and occlusion.15, 49, 55, 83, 93, 95 Fistulous communications may then be evident either proximal or distal to the site of obstruction.

Figure 5. The subepicardial coronary arteries are primarily involved with ventriculo-coronary arterial connections and the resulting process of myointimal hyperplasia. The epicardial involvement is evident from this low-power section of right ventricular myocardium stained with Movat pentachrome. The coronary arteries are remarkably thickened, and flow through them would certainly be compromised.

Figure 6. This panorama shows the histopathological changes in coronary arteries from patients with pulmonary atresia and an intact ventricular septum. The nature of the histopathological changes is discussed in the text. (a) Movat pentachrome stain. There is mild-to-moderate medial hyperplasia without important obstruction. (b) and (c) The Movat pentachrome stain shows more severe narrowing, with more important vascular dysplasia, myointimal hyperplasia, and deposition of ground substance. (d–f) The Movat pentachrome stain now shows very severe narrowing, to the point of virtual occlusion. The normal integrity of the vascular structure is lost. These changes in the coronary arteries were once attributed either to occlusive fibroelastosis, or to an inflammatory process. Both theories were later shown to be incorrect.
Much has now been written concerning the histology of the coronary arteries, with some of the observations dating back more than four decades.9–12, 14, 16, 17, 19, 20, 22, 74, 76 The changes in the walls of the coronary arteries fed by the ventriculo-coronary communications are not characterized by inflammation, as was once thought. Instead, the changes are more appropriately described as myointimal hyperplasia, set against a rich background of glycosoaminoglycans.9–12, 14, 16, 17, 19, 20, 22, 74, 76 The vascular pathology ranges from mild degrees of intimal and medial thickening, in which a continuous internal elastic lamina and normal lumen is present, to a loss of normal arterial mural morphology, with replacement of the arterial wall by fibrocellular tissue containing irregular, non-organized strands of elastin, and severe stenosis or complete occlusion of the arterial lumen (Figs 5 and 6). These changes have previously been characterized as “fibroelastosis” of the coronary arteries,11 but the emphasis is better placed on the myointimal hyperplasia14, 19, 20, 22 (Fig. 6b–6f). Staining for glycosaminoglycans shows the prominent formation of ground substance by the activated smooth muscle cells, rather than reduplication of elastic tissue and collagen as is typical for fibroelastosis. This pathologic process results in profound distortion of the normal coronary arterial architecture, eventuating in endothelial irregularity, stenosis, or interruption. These coronary arterial changes are observed only in the setting of ventriculo-coronary arterial connections, and by inference, a hypertensive right ventricle.
We have already commented on the changes of myocardial ischaemia and infarction identified in some of these patients9, 12, 14, 16–20, 72–76, 85–87, 96 (Fig. 7). Indeed, myocardial rupture secondary to acute infarction has been documented in the occasional patient.97 Other myocardial abnormalities have also been described (Fig. 7), including alleged myocardial noncompaction,14, 18–20, 85, 98 myocardial disarray,14, 18–20, 85, 99 and endocardial fibroelastosis.14, 18–20, 85–87 In the setting of a hugely dilated right ventricle, there is also marked thinning of the myocardium, but this change is not germane to any discussion of ventriculo-coronary arterial connections, since as we have emphasized, the communications with the coronary arterial system are virtually unknown when the right ventricle is dilated and its myocardium thinned. Bulkley et al.99 attributed the myocardial disarray to the prolonged periods of isometric contraction, which they argued must occur in some patients with aortic or pulmonary atresia and intact ventricular septum. Akiba and Becker100 were unable to confirm the claim regarding myocardial disarray. Instead, they found areas of acute myocardial ischaemia, with elevated levels of endomysial collagen, in some of their specimens. They speculated such disease involving the left ventricle could be a limiting factor for long-lasting successful intervention.100 We49 have seen some evidence of myocardial disarray, but such changes have not been frequent or conspicuous (Fig. 7j). Oosthoek et al.101 found an abnormal distribution of capillaries, along with abnormal myocytic morphology, and they also described myocardial disarray in some patients, most frequently in the setting of hypoplastic right ventricles and ventriculo-coronary arterial connections. Bulging of the septum into the left ventricle has been observed in a number of patients with small and hypertensive right ventricles38, 55 (Fig. 8). This may contribute to severe obstruction of the left ventricular outflow tract after volume-unloading surgery.102
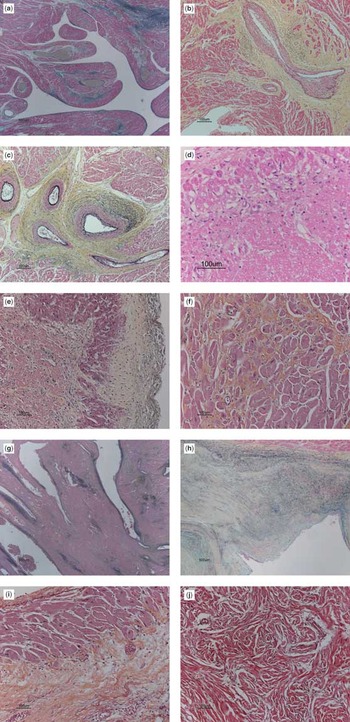
Figure 7. Types of myocardial changes observed in patients with pulmonary atresia, intact ventricular septum, and ventriculo-coronary arterial connections. (a) Elastic trichrome stain. Photomicrograph of the right ventriclular myocardium from a 3-month-old female with barium gelate infusate (the gray lakes) within the dilated subendocardial coronary arteries indicative of ventriculo-coronary arterial connections. (b) and (c) In a different patient, the Movat pentachrome stain shows considerable perivascular fibrosis (yellow staining tissue) and loss of normal myocardium. (d) A young baby with a recent infarction shows considerable myocytolysis in this section stained with Movat pentachrome. (e) The photomicrographs of this section of the left ventricle stained with Movat pentachrome show diffuse recent subendocardial necrosis, with sparing of the layers of myocardial cells immediately beneath the endocardium, the endocardial surface being visible to the right hand side of the panel. The immediate subendocardial myocardium receives blood by direct perfusion from the right ventricular cavity. (f) This section of right ventricular myocardium stained with Movat pentachrome shows hypertrophy of the myocardial cells, interstitial fibrosis, and loss of normal architecture, changes consistent with ischaemia. (g) This section shows an area of non-compaction, with endothelial-lined spaces. (h) The right ventricular endocardial surface stained with Movat pentachrome in an 8-month-old patient with pulmonary atresia and intact ventricular septum who had no ventriculo-arterial connections detected clinically or at the time of postmortem examination. The small right ventricle shows severe endocardial fibroelastosis. (i) The Movat pentachrome stain of the right ventricular myocardium shows extensive old ischaemic injury, with subendocardial replacement fibrosis in the lower portion of photo, with a thin layer of the immediate subendocardium being spared. Note the marked cardiomyocytic hypertrophy. (j) Myocardial disarray from interventricular septum. We have not, however, seen this as a particularly common feature in the setting of ventriculo-coronary arterial connections.

Figure 8. The left ventricular septal bulge in a patient with coronary arterial abnormalities and a hypertensive right ventricle. (a) Gross specimen showing the left ventricular outflow tract compromised by a striking septal bulge. The pallor of the subendocardial myocardium is evident (white arrows) consistent with ongoing ischaemia and fibrosis. AO: aorta; MV: mitral valve. (b) Long axial oblique left ventriculogram (LV) shows the septal bulge (white arrows) narrowing the left ventricular outflow tract (LVOTO).
The observations discussed above concerning the disordered coronary arterial circulation helped us, and others, to formulate the view that, in some patients, retrograde flow of blood from the right ventricle to the coronary arteries was necessary during systole to maintain some degree of myocardial perfusion, and thus viability. If the concept is correct, it follows that the right ventricular-dependent coronary arterial circulation should be unmasked by hindering or preventing this retrograde systolic flow, either by preventing blood from entering the right ventricle, or by reducing the systolic driving pressure. Several manoeuvres were subsequently shown to unmask the abnormal coronary arterial circulation, including:
Several abnormal variants of the coronary arteries necessitate systolic flow from the right ventricle so as to ensure an adequate coronary arterial circulation:

Figure 9. Atresia of the aortic origins of both coronary arteries in a patient with pulmonary atresia and intact ventricular septum. (a) Retrograde aortogram (AO) shows dense opacification of the aortic root without visualization of either coronary artery. (b) Frontal injection in a direct aorto-coronary collateral artery originating from the descending thoracic aorta (DAO) with subsequent opacification of the coronary artery and right ventricle (RV). Reproduced from Freedom et al., reference #128, with permission of Cardiology in the Young.

Figure 10. Atresia of the aortic origin of the left coronary artery in a patient with pulmonary atresia and intact ventricular septum, a very rudimentary right ventricle (RV), and obliteration of the infundibulum. This frontal right ventriculogram shows a densely opacified (white arrows) left anterior descending coronary artery filled via the ventriculo-arterial coronary connection (VCAC), but no reflux of contrast into the aortic root is appreciated (black arrow).

Figure 11. Panorama of important coronary arterial abnormalities demonstrated by angiography in patients with pulmonary atresia and intact ventricular septum. (a) Lateral right ventriculogram shows a very hypoplastic right ventricle (RV) with obliteration of the infundibulum and multiple levels of interruption and stenosis (white arrows) of the anterior descending coronary artery that does connect to the aorta (AO). LSV: left sinus of Valsalva. There are also multiple connections between the diminutive right ventricle and the involved coronary artery. (b) Injection of contrast into the left coronary sinus of the aorta (AO) shows an interruption in the mid-portion of the anterior descending coronary artery (LAD) with a normal appearing left circumflex coronary artery (LCX). (c) A mid-anterior descending coronary artery (LAD) interruption with important ectasia of this vessel is demonstrated by this lateral right ventriculogram (RV) with connection to the aorta (AO). The right ventricle is bizarre in shape. VCAC: ventriculocoronary arterial connection.
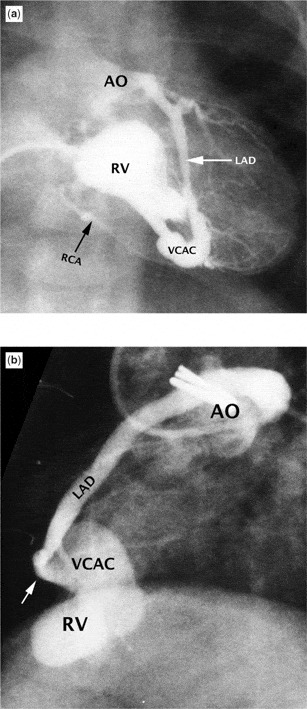
Figure 12. Panorama of less severe coronary arterial abnormalities in patients with pulmonary atresia and intact ventricular septum as shown by angiography. (a) Frontal right ventriculogram (RV) shows opacification of both the right (RCA) and anterior descending coronary arteries (LAD) with the left system clearly connecting with the aortic root (AO). The left anterior descending coronary artery is mildly dilated and ectatic. (b) A distal interruption (white arrow) of the left anterior descending coronary artery (LAD) is seen in this lateral right ventriculogram (RV). The connection (VCAC) between the right ventricle and coronary artery is quite dilated. Note the obvious connection with the aorta (AO).
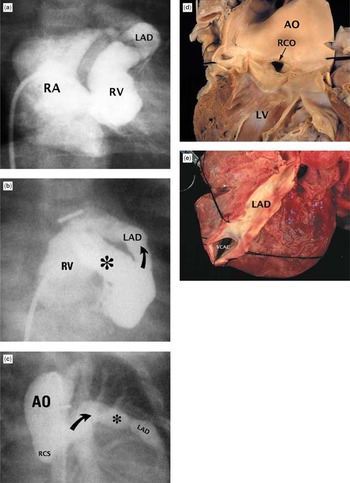
Figure 13. Massive coronary arterial-to-right ventricular fistulas in patients with pulmonary atresia and intact ventricular septum. (a) frontal angiogram showing small right ventricle (RV), large right atrium (RA) with opacification of a severely dilated left anterior descending coronary artery (LAD). (b) Another patient whose frontal right ventriculogram shows a huge connection (*) to a much enlarged left anterior descending coronary artery (LAD). Curved arrow shows direction of flow in this frame. (c) Another patient whose frontal aortogram shows a very dilated left coronary artery, but there is no coronary artery originating from the right coronary sinus (RCS). The asterisk notes some ectasia of the enlarged coronary artery. (d) Internal view of left ventricle (LV) and aorta (AO) in a patient with a very dilated right coronary artery. (e) The same patient as shown in (d). Note the very dilated left anterior descending coronary artery (LAD) and the connection (VCAC) to the right ventricle.
The abnormal circulatory pattern can also be produced by anomalous origin of the right coronary artery from the pulmonary trunk, a lesion described in the setting of pulmonary atresia and intact ventricular septum, albeit that it does not require systolic flow of blood from the right ventricle.114 Another patient with pulmonary atresia and intact ventricular septum has been described with a single coronary artery originating from the left-posterior dimple of the blind pulmonary root.115 This coronary artery branched into an anterior interventricular artery, a left circumflex artery, and a right coronary artery. Both the right coronary artery and the inferior interventricular artery had fistulous connections to the right ventricle. The same abnormal circulatory pattern can also be produced by combinations of the lesions listed above.
From the above possibilities, we can conclude that the most egregious form of the right ventricular-dependent coronary arterial circulation is that in which both coronary arteries lack their normal proximal connections with the aorta106–110 (Fig. 9). In this setting, flow into the coronary arteries is mandated exclusively by right ventricular events. In terms of severity, this is probably followed by atresia of the aortic orifice of the main stem of the left coronary artery, and then by the others as listed above. There is, nonetheless, a spectrum of coronary arterial pathology, as demonstrated both at necropsy or by angiographic imaging. It is also well established that mild obstructions, with time, can progress to more severe pathology14, 15, 18, 21, 55, 83, 93–95, 116 (Figs 9–13). In one most unusual patient, who lacked proximal connections between the aorta and the coronary arteries, the systemic connection to the coronary arterial circulation was via an unusual collateral artery taking its origin from the descending thoracic aorta108 (Fig. 9).
We have already made comment concerning the extensive literature devoted to the angiocardiographic imaging of coronary arterial anatomy.14, 15, 18, 21, 55, 83, 95–97, 116 Based on our long and substantial experience with this disorder, we use the working rule that, if selective right ventriculography fails to demonstrate connections with a coronary artery, it is very unlikely that there will be significant pathology involving that artery.55 The rare situation when a single coronary artery, or a right coronary artery, originates from the pulmonary trunk, nonetheless, must always be remembered.114, 115 If there is opacification of any portion of the coronary arterial system from the right ventriculogram, then it is essential also clearly to image the coronary arteries if the situation is not clarified by the ventriculogram. Such further definition of the coronary arterial circulation can be achieved in any of a number of ways,55 including balloon occlusion aortography, retrograde aortography with or without selective coronary arteriography, or selective coronary arteriography from the venous or transatrial approach.117 In addition to determining whether a coronary artery is stenotic, and establishing the degree of obstruction or site of any interruption, it is important to ascertain that the involved coronary arteries retain their patent origins from the aorta (see Figs 1 and 9–12). As we have already discussed, patients have been described when both coronary arteries, or the left coronary artery, lack their normal patent proximal origin from the aorta106–112 (Figs 9 and 10). When a coronary artery is opacified densely by right ventriculography, reflux of contrast material into the aortic root should be anticipated, thus confirming the patency of the proximal connections.55 If such reflux is not apparent (see Figs 1 and 9–12), then other studies are necessary to rule out atresia of the proximal coronary arterial origins. Serial angiographic studies have shown progression of coronary arterial pathology.14, 15, 18, 21, 55, 83, 95–97, 116
More recently, echocardiography has been shown to be effective in defining the coronary arterial circulation.118–121 A great deal of information can be gleaned from a careful and systematic echocardiographic examination, including recognition of ventriculo-coronary connections in the fetus. In one retrospective study,120 which compared angiographic and echocardiographic findings, it was shown that the echocardiographically-derived tricuspid valve Z-score predicted both the likelihood of coronary arterial fistulas, and the presence of a right ventricular-dependent coronary arterial circulation.120 The authors of this report120 argued that their observations could rationalize the need for diagnostic catheterization. One of the obvious drawbacks of the study was its retrospective nature, as noted by the authors themselves.120 Although the paper was accepted for publication several years ago, we are unaware of any subsequent prospective study that has used similar methodologies. As might be anticipated, there are also exceptions to these conclusions. We, and others, have seen right ventricles of normal size supporting a right ventricular-dependent coronary arterial circulation, and conversely, a diminutive right ventricle with normal coronary arterial anatomy.55, 84 It is still likely that all patients being considered for either a biventricular or a one-and-a-half ventricular repair will undergo catheterization, with the intention to perform catheter perforation of the atretic pulmonary valve. Should any significant ventriculo-coronary arterial connections be present, they will be demonstrated by a right ventriculogram performed at this time. At the present time, therefore, although cognizant of the value of echocardiography, our practice is still to perform catheterization, with appropriate angiographic imaging of the coronary arterial circulation, so as to provide optimal information for determining treatment, prognosis, and counselling.
Determination of the prevalence of any specific congenital cardiac malformation depends on the era in which the calculation was made, and the methodologies used in the determination. These potential caveats, particularly because determination of ventriculo-coronary arterial communications and the right ventricular dependent circulation is qualitative and debateable, make the definition of their incidence even more difficult. To obtain these data, it is first necessary to determine the incidence of pulmonary atresia with an intact ventricular septum. Having determined this population, it is then necessary to define the incidence of ventriculo-coronary arterial connections. Only then is it possible to establish the proportion having a right ventricular-dependent coronary circulation. Data published in 1980 from the Report from the New England Regional Infant Cardiac Program showed that the disorder, accounted for 3.3 percent of all babies entered into the study.122 The more recent Baltimore-Washington Infant Study calculated the prevalence of pulmonary atresia and intact ventricular septum to be 0.083 per 1,000 live births.123 The prospective Bohemian Survival study,124 published in 2000, produced a prevalence of 0.06 per 1,000 live-births, with the malformation accounting for 1.05 percent of all cardiac malformations surveyed in this study. Data provided by the Pediatric Cardiac Care consortium showed that the malformation accounted for 2.6 percent of all patients surveyed.125 This study showed that, in Arkansas, the prevalence was 0.065 for each 1,000 live births, but was 0.085 for Iowa, and 0.058 for Minnesota. The incidence in the United Kingdom and Eire was 4.5 cases for each 100,000 live-births, as based on a survey conducted from 1991 to 1995.126 Between 1980 and 1995, 29 patients had been identified in one health region of the United Kingdom from a birth cohort of 601,635 live-births, giving a prevalence of 0.049 per 1,000 live-births.127
What, then, is the incidence of ventriculo-coronary arterial connections? Review of some 13 series reported in the literature suggests that they are present in two-fifths of cases, albeit that the incidence varies from a low of less than one-tenth to an upper value of more than one-half. From these series,23, 27, 28, 30–33, 93, 94, 96, 125, 128, 129 we calculated that just over one-fifth of patients had a right ventricular-dependent coronary circulation.
The platform begins with all those many developments that have enhanced in recent years the medical and surgical care of the neonate. These have been summarized elsewhere.83 Over the past four decades, nonetheless, several initiatives and observations have been especially important for the surgical management, and hence improved outcomes, for patients specifically with pulmonary atresia and an intact ventricular septum. These include the introduction of the Fontan operation in 1971,130 its many subsequent modifications, the role of prostanoid therapy in maintaining ductal patency,131 and the extensive findings relative to the disturbed cardiac anatomy, in particular the recognition of the right ventricular-dependent coronary arterial circulation and its implications for surgical management.8, 13, 14, 18–22 Also important have been the introduction of the bidirectional cavopulmonary shunt in staging to completion of the Fontan circulation, along with the emergence of the one-and-a-half ventricle repair.132 The one-and-a-half ventricle repair, however, is not germane to the patient whose coronary circulation is in large part right ventricular-dependent, nor is catheter-based therapy. Functionally univentricular palliation, in contrast, certainly did salvage some of the patients with a right ventricular-dependent coronary arterial circulation.132–134
Large collaborative studies, including those performed by the Congenital Heart Surgeons Society,28, 33 the British Paediatric Cardiac Association,126 and the Paediatric Cardiac Care Consortium,125 have been very helpful in clarifying those variables, anatomical and otherwise, that influence the outcomes. Similar benefits have accrued from examination of outcomes achieved in single institutions that have accumulated large cohorts of patients.23, 24, 30–32 For those whose coronary circulation is in large part right ventricular-dependent, it is the recognition of this fact, and the institution of functionally univentricular palliation, that has salvaged at least some of them.28–33, 95, 133, 134 Even to the mid-1980s, diagnostic algorithms for such patients often failed to include the coronary arterial anatomy. In the same era, surgical algorithms were frequently constructed on the basis of various morphological features, including some determination of right ventricular size, or the presence or absence of one or more components of the morphologically right ventricle, but again usually in the absence of any discussion of pertinent coronary arterial pathology.92, 135, 136 Coincident with evolving understanding of the nature of the disorder, and particularly the disturbed coronary arterial circulation, there has been an evolution in the description of hearts with this disorder. The descriptions employed, and the measurements used in categorization, reflect the profound heterogeneity of the morphological variables. Most categorizations have been descriptive or semi-quantitative, and none have been universally adopted, albeit that many now do employ the tricuspid valve both as a measure of right ventricular size and as a predictor of ventriculo-coronary arterial connections.28, 33 It has been suggested that determination of the volume of the right ventricle would be useful,136 but because of the often unusual shape of the right ventricle, this methodology has not proved popular.
Intrauterine death of fetuses with pulmonary atresia and intact ventricular septum has been well catalogued.83 A number of studies have addressed prenatal recognition, most focusing on those fetuses with severe tricuspid insufficiency, albeit with some reports describing ventriculo-coronary arterial connections.137–142 Fetuses with florid tricuspid regurgitation, and a large, dilated, and thin-walled right ventricle, are known not to fare well.83 As we have emphasized, however, such fetuses do not have ventriculo-coronary arterial connections. Rarely, the fetus with a hypertensive right ventricle and a tenuous coronary artery circulation may experience progressive left ventricular dysfunction, with subsequent fetal death.143 In the series reported by Sharland et al.139 nearly three-fifths of the mothers in whom the fetus had been shown to have a small right ventricle elected to terminate the pregnancy. Of those pregnancies that continued, a significant proportion died either during the ongoing gestation or in the neonatal period. The outlook for those in this group with a dilated right ventricle and gross tricuspid regurgitation was also terrible, with over half of the families choosing termination of pregnancy; and the others either dying during ongoing gestation or in the neonatal period. Part of the study conducted on behalf of the British Paediatric Cardiac Association126 was designed to address the impact of fetal echocardiography on the incidence of pulmonary atresia and intact ventricular septum at birth, and on postnatal outcome. Without fetal diagnosis and termination of pregnancy, or loss during gestation, the live-born incidence would have been 5.6 per 100,000 births in England and Wales, 5.3 in Scotland, but unchanged in Eire and Northern Ireland. We can conclude, therefore, that spontaneous fetal death, and therapeutic abortion, certainly reduce the incidence of live-born babies with pulmonary atresia and intact ventricular septum, particularly those with severe tricuspid regurgitation. There is less evidence, however, that spontaneous fetal loss reduces the live-born prevalence of babies with the hypertensive form of pulmonary atresia and intact ventricular septum and ventriculo-coronary arterial connections.
The majority of babies depend for survival on patency of the arterial duct. A few remarkable patients have survived for a number of years without palliation, but this is most uncommon.144, 145 There is a relatively sparse literature addressing survival of patients with pulmonary atresia from a specific geographic area, and much of the data in these reports includes patients undergoing surgical procedures. Outcomes for these patients from diverse geographical regions of the world reflect a tremendous number of variables. For patients born with congenital cardiac disease in Bohemia between 1980 and 1990,124 only just less than one-fifth of those with pulmonary atresia and intact ventricular septum survived beyond the first year of life. Of the children born with pulmonary atresia and intact ventricular septum in Sweden from 1980 to 1999,146 seven-eighths underwent surgery, and three-quarters of these survived at one year, albeit that this included only half of those with ventriculo-coronary arterial connections. Of these 36 patients, 8 were considered to have a right ventricular-dependent coronary circulation. The natural and unnatural history of patients born with pulmonary atresia between 1980 and 1995 has been well documented for the Northern Health Region of England, a geographically well-defined area with a population of about three million.127 Of the 129 patients identified with pulmonary atresia, 29 had pulmonary atresia and intact ventricular septum, and just over half of these had died in the first year of life. No information, however, was provided about the presence or absence of ventriculo-coronary arterial connections in these patients. It is of interest, nonetheless, that three patients died suddenly, and that these sudden deaths could not be explained by autopsy examination, suggesting the possibility of an arrhythmia.
Our own earlier publications have documented the dismal experience in Toronto with palliation of modest numbers of infants in the years between 1950 and 1969.79, 83 After 1976, with the introduction of prostanoid therapy,131 surgical results began to improve, and more radical operations were introduced to relieve the obstructed right ventricular outflow tract.134 Just about two decades ago, Corno et al.147 called attention to the deleterious effects of simultaneous patency of both a systemic-to-pulmonary arterial shunt and an arterial duct, the latter maintained by prostanoid therapy. They suggested that simultaneous patency of the shunt and the duct would contribute to myocardial failure, presumably by lowering the aortic diastolic pressure and compromising flow of blood to the coronary arteries in an already volume-loaded left ventricle. While they did not specifically mention the role of an intrinsically abnormal coronary circulation, there is little doubt that the dynamics of coronary arterial flow would be very precarious in this situation. Already by 1989, the group from Toronto23, 24 had identified ventriculo-coronary arterial connections with a right ventricular-dependent coronary circulation as risk factors for poor outcomes for patients undergoing an attempted biventricular repair. This finding was confirmed several years later by Hanley et al.28 reporting for the Congenital Heart Surgeons Society. They showed that univentricular palliation permitted equivalent outcomes to be achieved in patients with small right ventricles and or coronary arterial abnormalities. Although accepting that a biventricular repair is preferable to functionally univentricular palliation, we agree with Ashburn et al.33 who wrote: “Selecting a Fontan pathway for children with marginal or severe anatomy should not be considered a failure of therapy”. This notion is now endorsed by the increasing numbers of patients surviving construction of the Fontan circulation.30–33 Several contemporary reports have documented remarkable improvement in survival in substantial numbers of patients with pulmonary atresia and intact ventricular septum, ranging from 76 percent to 98 percent at 5 years.30–33 Despite excellent survival, however, the proportion of patients undergoing definitive repair in these series varied only from 55 percent to 72 percent.30–33
Few have investigated the outcomes only in patients with a right ventricular-dependent coronary circulation.148 It is noteworthy, therefore, that Powell et al.149 reported on 12 such patients seen at the Children's Hospital in Boston between 1986 and 1997. At the time their paper was published, 5 of the patients had undergone a fenestrated Fontan procedure, 3 were awaiting the procedure, 1 patient had died intraoperatively during construction of a modified Blalock-Taussig shunt, one died suddenly at 4 months of age, and one patient had undergone a classic Glenn shunt along with an aorto-left pulmonary artery shunt. The known mortality, therefore, was 2 of 12 patients (16.7 percent). In the more recent study of over 200 patients seen at the Toronto Hospital for Sick Children from 1965 to 1998,23 a right ventricular-dependent coronary circulation was identified in just under one-quarter of the cohort. The overall survival for the entire cohort was 72 percent at 1 month; 57 percent at 1 year, and 50 percent at 5 to 23 years. When stratified into four groups by era, overall survival was much improved in the most recent cohort, with survival of nearly 90 percent at 5 years and beyond, comparable to other current studies. Ventriculo-coronary arterial connections, and a right ventricular-dependent coronary circulation, an important risk factor in our earlier surgical experience,23, 24 therefore, had been effectively neutralized by the introduction of functionally univentricular palliation.
For historical interest, note should be taken of an interesting and novel approach, mentioned by de Leval150 in a book chapter published in 1986, and attributed by him to James Sink. Sink had suggested that, if suprasystemic right ventricular pressure is disadvantageous, the right ventricular pressure could be equilibrated to systemic levels by interposing a conduit between the right ventricle and the aorta. According to de Leval,150 this procedure was carried out in two patients, one of whom survived. Some years later, in 1993, Freeman et al.151 reported success with this procedure in another patient. The theoretical advantages of the manoeuvre include reduction of the magnitude of right ventricular hypertrophy by reduction of suprasystemic to systemic levels of right ventricular pressure, reduction in the myocardial substrate for ventricular arrhythmias, and reduction of turbulent flow at the junction of the ventriculo-coronary communications.151 This last factor may reduce the predisposition to myocardial ischaemia. Admittedly, these are all theoretical advantages. This experience, nonetheless, was extended by Laks et al.152 They reported experience in 5 patients, all of whom survived the surgery without showing evidence of myocardial ischaemia. There is little, if any, recent literature, however, suggesting widespread application of this technique.
The technique used for construction of the Fontan procedure in patients with a right ventricular-dependent coronary circulation may also be important.132–134, 153 Cardiopulmonary bypass unloads the right ventricle, and thus could compromise the supply of blood to the portion of the myocardium that is right ventricular-dependent. In order to obviate this situation, many are now performing an extracardiac Fontan procedure without cardiopulmonary bypass, thus hoping to prevent any adverse coronary arterial events.132–134, 153
Interim mortality amongst patients with hypoplasia of the left heart syndrome undergoing staged univentricular palliation has been summarized elsewhere.154 There is considerably less literature addressing interim mortality for patients with pulmonary atresia and intact ventricular septum, especially those undergoing functionally univentricular palliation. Some of this information can be surmised or inferred from the larger collaborative series, or those describing experience in a single institution.28–33 Significant interim death in infants with pulmonary atresia and a hypoplastic right ventricle was reported by Fenton et al.155 in a retrospective study. Their study, combining data from two institutions, showed that interim mortality was higher for infants with pulmonary atresia with intact ventricular septum than those with hypoplasia of the left heart if account is taken only of those with severe right ventricular hypoplasia. They suggest that the rate of interim death should be considered when contemplating different options for treatment, such as shunting versus transplantation. The most common cause of death in their study was attributed to abnormal myocardial perfusion, focusing attention once more on the often abnormal coronary arterial circulation in this population of patients.155
There is no doubt that the Fontan operation has offered considerable benefit to some patients whose cardiac malformation is not amenable to either a biventricular or a one-and-a-half ventricle repair. We have discussed elsewhere those factors that make perfect construction of the Fontan circulation an unlikely situation.156, 157 Although one can take satisfaction that many patients with a right ventricular-dependent coronary circulation can be managed on a protocol of functionally univentricular palliation, we must still acknowledge that the coronary circulation in these patients is often tenuous, and that ongoing turbulent flow, with accelerated shear forces, into already disadvantaged coronary arteries will further compromise their vascular integrity. This has the potential for promoting or enhancing myocardial ischaemia, and producing the substrate for ventricular arrhythmias. Sudden cardiac death is a well-documented phenomenon in some patients with pulmonary atresia and intact ventricular septum, and can likely be attributed to an ischaemic myocardium.146, 155 Hausdorf et al.158 found that patients with ventriculo-arterial coronary connections showed disturbances of regional wall motion. They found a high degree of coincidence between the disturbances of regional wall motion and the topography of myocardial perfusion from persisting fistulous communications. Since all of these patients had ventriculo-coronary arterial connections, these authors suggest their findings likely reflect apical left ventricular ischaemia.158 It was also shown that our own patients in Toronto with ventriculo-arterial coronary connections and coronary abnormalities have a higher incidence in abnormalities of wall motion, again likely reflecting ongoing ischaemia, and that such patients were at risk for late death.128 Others have found that some patients with pulmonary atresia and intact ventricular septum, primarily those palliated with a systemic-to-pulmonary arterial shunt, have impaired left ventricular function and a less compliant left ventricle.159 It has also been shown that, after construction of a bidirectional Glenn procedure and a total cavopulmonary connection, contractility and ventricular efficiency of patients with pulmonary atresia with intact ventricular septum are inferior to those of patients with tricuspid atresia.160 Based on these findings, it is suggested that a high-pressure residual right ventricle, with an ischaemic myocardial substrate, may impair the left ventricular performance after cavopulmonary surgery.161 Patients surviving any of the stages of functionally univentricular palliation, therefore, should be surveyed for life-threatening ventricular arrhythmias. The myocardial health of these patients should be evaluated using any or all of the currently available methodologies. While relatively straight-forward methodologies, such as echocardiographic evaluation of ventricular function and assessment of abnormalities of left ventricular wall motion, are important, information about myocardial viability, metabolism, and coronary reserve gleaned from positron-emission tomography should also be important. Despite these long-term concerns about the state of myocardial perfusion in these patients, it is gratifying to note the excellent quality of life perceived by children with pulmonary a tresia and intact ventricular septum who have undergone surgery.161
Pulmonary atresia with an intact ventricular septum is a morphologically heterogeneous disorder. Ventriculo-coronary arterial connections are present in a substantial number of patients with this malformation, potentially complicating surgical management. In the nearly eight decades since the earliest description of the connections in these patients, we have come to learn that they might jeopardize a normal coronary circulation. Numerous abnormalities have been catalogued as underscoring the potential to produce a right ventricular-dependent coronary arterial circulation. The clinical inference to be made for patients with this abnormal pattern is that they are best managed by functionally univentricular palliation. The introduction of this approach has neutralized in large part the dismal outlook for these patients, since they were typically managed previously by attempted conversion to a biventricular circulation.
We thank Ms Ruth Taylor, of the Division of Cardiology, for her excellent secretarial support.
The research on which this review is based was supported by grants from the British Heart Foundation together with the Joseph Levy Foundation.
Research at the Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust benefits from R&D funding received from the NHS Executive.

Ventriculo-coronary arterial connection in pulmonary atresia and intact ventricular septum. (a) Frontal right ventriculogram shows a hypoplastic right ventricle with attenuation of all three components of its cavity. Both the right (RCA) and the anterior descending coronary artery (LAD) clearly connect with the aortic root (AO). The right ventricle (RV) also connects via a ventriculo-coronary connection (curved arrow) with the coronary circulation. The white arrows show mild narrowing in the anterior descending coronary artery. The right coronary artery (black arrows) is mildly dilated. (b) The lateral projection of the injection showed in (a) shows ectasia and narrowing of the anterior descending coronary artery (white arrows). The connection between the right ventricle and the coronary circulation is marked by the black arrow. (c) A hematoxylin and eosin stain shows a ventriculo-coronary arterial connection (VCAC) between the cavity of the right ventricle (RV) and a coronary artery.

External view of the heart from a patient with pulmonary atresia and intact ventricular septum. The aorta (AO) is dilated, and the proximal pulmonary trunk (MPA) is represented by a fibrous cord (two small black arrows). The aortic arch is left-sided and the arterial duct (DA) supplies the confluent pulmonary arteries. The epicardial course of the left anterior descending coronary artery (LAD) demarcates a larger left ventricle (LV) from the smaller right ventricle (RV). The left anterior descending coronary artery originates from the dilated, ecstatic, and thickened right coronary artery (RCA).

This specimen from a patient with pulmonary atresia and intact ventricular septum is dissected to demonstrate the massive hypertrophy of the ventricular septum and the right ventricular free-wall (RVFW). Note the difference in thicknesses between the right and left ventricles (LV). The subedocardial myocardium is pale, consistent with reduced perfusion and likely chronic ischaemic changes. The tricuspid valve (TV) is appreciably smaller than the mitral valves (MV). AO: aorta; RA: right atrium.

The lateral right ventriculogram (RV), using a double-catheter technique, reveals absence of the infundibulum of the right ventricle. MPT: pulmonary trunk.

The subepicardial coronary arteries are primarily involved with ventriculo-coronary arterial connections and the resulting process of myointimal hyperplasia. The epicardial involvement is evident from this low-power section of right ventricular myocardium stained with Movat pentachrome. The coronary arteries are remarkably thickened, and flow through them would certainly be compromised.

This panorama shows the histopathological changes in coronary arteries from patients with pulmonary atresia and an intact ventricular septum. The nature of the histopathological changes is discussed in the text. (a) Movat pentachrome stain. There is mild-to-moderate medial hyperplasia without important obstruction. (b) and (c) The Movat pentachrome stain shows more severe narrowing, with more important vascular dysplasia, myointimal hyperplasia, and deposition of ground substance. (d–f) The Movat pentachrome stain now shows very severe narrowing, to the point of virtual occlusion. The normal integrity of the vascular structure is lost. These changes in the coronary arteries were once attributed either to occlusive fibroelastosis, or to an inflammatory process. Both theories were later shown to be incorrect.

Types of myocardial changes observed in patients with pulmonary atresia, intact ventricular septum, and ventriculo-coronary arterial connections. (a) Elastic trichrome stain. Photomicrograph of the right ventriclular myocardium from a 3-month-old female with barium gelate infusate (the gray lakes) within the dilated subendocardial coronary arteries indicative of ventriculo-coronary arterial connections. (b) and (c) In a different patient, the Movat pentachrome stain shows considerable perivascular fibrosis (yellow staining tissue) and loss of normal myocardium. (d) A young baby with a recent infarction shows considerable myocytolysis in this section stained with Movat pentachrome. (e) The photomicrographs of this section of the left ventricle stained with Movat pentachrome show diffuse recent subendocardial necrosis, with sparing of the layers of myocardial cells immediately beneath the endocardium, the endocardial surface being visible to the right hand side of the panel. The immediate subendocardial myocardium receives blood by direct perfusion from the right ventricular cavity. (f) This section of right ventricular myocardium stained with Movat pentachrome shows hypertrophy of the myocardial cells, interstitial fibrosis, and loss of normal architecture, changes consistent with ischaemia. (g) This section shows an area of non-compaction, with endothelial-lined spaces. (h) The right ventricular endocardial surface stained with Movat pentachrome in an 8-month-old patient with pulmonary atresia and intact ventricular septum who had no ventriculo-arterial connections detected clinically or at the time of postmortem examination. The small right ventricle shows severe endocardial fibroelastosis. (i) The Movat pentachrome stain of the right ventricular myocardium shows extensive old ischaemic injury, with subendocardial replacement fibrosis in the lower portion of photo, with a thin layer of the immediate subendocardium being spared. Note the marked cardiomyocytic hypertrophy. (j) Myocardial disarray from interventricular septum. We have not, however, seen this as a particularly common feature in the setting of ventriculo-coronary arterial connections.

The left ventricular septal bulge in a patient with coronary arterial abnormalities and a hypertensive right ventricle. (a) Gross specimen showing the left ventricular outflow tract compromised by a striking septal bulge. The pallor of the subendocardial myocardium is evident (white arrows) consistent with ongoing ischaemia and fibrosis. AO: aorta; MV: mitral valve. (b) Long axial oblique left ventriculogram (LV) shows the septal bulge (white arrows) narrowing the left ventricular outflow tract (LVOTO).

Atresia of the aortic origins of both coronary arteries in a patient with pulmonary atresia and intact ventricular septum. (a) Retrograde aortogram (AO) shows dense opacification of the aortic root without visualization of either coronary artery. (b) Frontal injection in a direct aorto-coronary collateral artery originating from the descending thoracic aorta (DAO) with subsequent opacification of the coronary artery and right ventricle (RV). Reproduced from Freedom et al., reference #128, with permission of Cardiology in the Young.

Atresia of the aortic origin of the left coronary artery in a patient with pulmonary atresia and intact ventricular septum, a very rudimentary right ventricle (RV), and obliteration of the infundibulum. This frontal right ventriculogram shows a densely opacified (white arrows) left anterior descending coronary artery filled via the ventriculo-arterial coronary connection (VCAC), but no reflux of contrast into the aortic root is appreciated (black arrow).

Panorama of important coronary arterial abnormalities demonstrated by angiography in patients with pulmonary atresia and intact ventricular septum. (a) Lateral right ventriculogram shows a very hypoplastic right ventricle (RV) with obliteration of the infundibulum and multiple levels of interruption and stenosis (white arrows) of the anterior descending coronary artery that does connect to the aorta (AO). LSV: left sinus of Valsalva. There are also multiple connections between the diminutive right ventricle and the involved coronary artery. (b) Injection of contrast into the left coronary sinus of the aorta (AO) shows an interruption in the mid-portion of the anterior descending coronary artery (LAD) with a normal appearing left circumflex coronary artery (LCX). (c) A mid-anterior descending coronary artery (LAD) interruption with important ectasia of this vessel is demonstrated by this lateral right ventriculogram (RV) with connection to the aorta (AO). The right ventricle is bizarre in shape. VCAC: ventriculocoronary arterial connection.

Panorama of less severe coronary arterial abnormalities in patients with pulmonary atresia and intact ventricular septum as shown by angiography. (a) Frontal right ventriculogram (RV) shows opacification of both the right (RCA) and anterior descending coronary arteries (LAD) with the left system clearly connecting with the aortic root (AO). The left anterior descending coronary artery is mildly dilated and ectatic. (b) A distal interruption (white arrow) of the left anterior descending coronary artery (LAD) is seen in this lateral right ventriculogram (RV). The connection (VCAC) between the right ventricle and coronary artery is quite dilated. Note the obvious connection with the aorta (AO).

Massive coronary arterial-to-right ventricular fistulas in patients with pulmonary atresia and intact ventricular septum. (a) frontal angiogram showing small right ventricle (RV), large right atrium (RA) with opacification of a severely dilated left anterior descending coronary artery (LAD). (b) Another patient whose frontal right ventriculogram shows a huge connection (*) to a much enlarged left anterior descending coronary artery (LAD). Curved arrow shows direction of flow in this frame. (c) Another patient whose frontal aortogram shows a very dilated left coronary artery, but there is no coronary artery originating from the right coronary sinus (RCS). The asterisk notes some ectasia of the enlarged coronary artery. (d) Internal view of left ventricle (LV) and aorta (AO) in a patient with a very dilated right coronary artery. (e) The same patient as shown in (d). Note the very dilated left anterior descending coronary artery (LAD) and the connection (VCAC) to the right ventricle.