Digoxin toxicity has been a long-standing potential complication associated with digoxin therapy in adults and children.Reference Gotsman and Schrire 1 , Reference Kanji and MacLean 2 Although the description and aetiology of digoxin toxicity in adults has been well-documented, the literature describing digoxin toxicity in children is limited. In adults, clinical signs and symptoms of digoxin toxicity include anorexia, nausea, vomiting, diarrhoea, abdominal pain, headache, dizziness, confusion, atrial tachycardias, and other more worrisome ventricular tachyarrhythmias.Reference Kanji and MacLean 2 In children, however, symptoms may vary due to differences in age-related symptomatology, existing co-morbidities, inability to communicate symptoms, or other disease processes that mimic digoxin toxicity.Reference Wells, Young and Kearns 3 Causality also differs between adults and children. Digoxin toxicity in adults is commonly associated with altered digoxin distribution and elimination from renal insufficiency, polypharmacy, or other co-morbidities, whereas accidental digoxin ingestion and errors in dosing have frequently been reported as aetiologies for digoxin toxicity in children.Reference Berkovitch, Akilesh and Gerace 4 – Reference Iacuone 7
Measuring serum digoxin concentrations may be useful in determining digoxin toxicity, as has been described in the large amount of literature devoted to this patient in adults.Reference Kanji and MacLean 2 , Reference Ordog, Benaron, Bhasin, Wasserberger and Balasubramanium 8 , Reference Pickett and Dickinson 9 For treatment of congestive heart failure, levels between 0.5 and 1.0 ng/ml are recommended.Reference Hunt, Abraham and Chin 10 For heart rate control during atrial fibrillation, plasma levels are less-defined and are generally titrated to a specific heart rate goal. Typically, digoxin levels are considered therapeutic for heart rate control between 1.0 and 2.0 ng/mlReference Hunt, Abraham and Chin 10 and toxic when they exceed 2 ng/ml;Reference Ordog, Benaron, Bhasin, Wasserberger and Balasubramanium 8 however, patients may exhibit symptoms associated with digoxin toxicity with serum digoxin concentrations below this toxic level.Reference Ordog, Benaron, Bhasin, Wasserberger and Balasubramanium 8
In children, data describing the association between serum digoxin concentrations and signs and symptoms of digoxin toxicity are scarce.Reference Koren and Parker 11 , Reference Rutledge 12 The utility of serum digoxin concentrations in evaluating digoxin toxicity is unknown in children. Therefore, the primary aim of our study was to elucidate the relationship between toxic range serum digoxin concentrations (>2 ng/ml) and the symptomatology traditionally associated with digoxin toxicity.Reference Kanji and MacLean 2 We hypothesised that signs and symptoms of digoxin toxicity would be associated with toxic serum digoxin concentrations (>2 ng/ml) in children.
Methods
After obtaining the Institutional Review Board’s approval, we conducted a retrospective chart review of all children and adolescents <19 years of age who had received digoxin and had serum digoxin concentrations assessed between January, 2007 and June, 2013 at the Texas Children’s Hospital. Patients with accidental ingestion were excluded. Data collection included patient demographics, digoxin dose, indication for digoxin therapy, serum digoxin concentration levels and indication for levels, any signs and/or symptoms of digoxin toxicity, serum electrolytes, electrocardiograms, and patient co-morbidities. For patients who had multiple serum digoxin concentrations levels assessed, the encounter with the highest level was chosen for the evaluation. Patient charts were reviewed by two pharmacists for signs and symptoms associated with digoxin toxicity, including nausea, vomiting, anorexia, visual changes, mental status changes – including confusion, dizziness, drowsiness, or giddiness – and/or arrhythmias. Electrocardiograms were evaluated by a paediatric electrophysiologist for electrocardiographic changes associated with digitalis effect and digoxin toxicity, as previously described in the literature.Reference Fisch and Knoebel 13 – Reference Saner, Lange, Pierach and Aeppli 15 Findings included, but were not limited to, prolongation of the PR and RR intervals, ST segment depression, decrease in T wave amplitude, altered automaticity (depressed or enhanced), ectopic rhythms, and impaired conduction. For patients who had a baseline electrocardiogram available, comparisons with the electrocardiogram at the time of serum digoxin concentration assessment were carried out to determine whether electrocardiogram changes consistent with digoxin toxicity were present. All chart and electrocardiogram reviewers were blinded to the serum digoxin concentrations.
Descriptive statistical methods (mean, standard deviation) were used to characterise the study population. Student’s t-test and Fisher’s exact test were used to identify differences in patients with toxic serum digoxin concentrations and those without toxic serum digoxin concentrations. Serum digoxin concentrations were broken down into the following three categories – >2, 1–2, and <1 ng/ml – and graphical methods were used to determine relationships between increasing serum digoxin concentrations and digoxin toxicity. A p-value of <0.05 was determined to be statistically significant a priori. All analyses were performed using Stata v.12 (StataCorp, College Station, Texas, United States of America).
Results
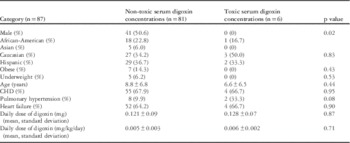
There were 87 patients who met the study criteria (male 47.1%). These patients had a median age of 10.4 years (interquartile range 0.9–14.5 years). Patients were racial and ethnically diverse, with 35% Caucasians (n=30), 36% Hispanics (n=31), 22% African-Americans (n=19), and 6% Asians (n=5). CHD was present in 67.8% (n=59) of the patients. Pulmonary hypertension was present in 11.5% (n=10) of the patients. The most common indication for digoxin toxicity was heart failure symptoms (64.4%, n=56).
The mean prescribed digoxin daily dose was 0.122±0.09 mg (0.005±0.004 mg/kg/day). Serum digoxin concentrations were obtained to rule out digoxin toxicity in 72.5% (n=63); the remainder (27.5%, n=24) had levels assessed as routine surveillance. The mean serum digoxin concentration was 1.01±0.71 ng/ml. An electrocardiogram was performed in 72.4% (n=63) of patients who had serum digoxin concentrations assessed, and only three of the six (50%) patients with a toxic serum digoxin concentrations had an electrocardiogram performed at that time. No patients received Digoxin Immune Fab.
Toxic levels (>2.0 ng/ml) were present in 6.9% (n=6) of the population (mean 2.6±0.6 ng/ml). The presence of signs and symptoms of digoxin toxicity was not different between patients with toxic serum digoxin concentrations and those without toxic serum digoxin concentrations when evaluating nausea/anorexia (3.7 versus 0%, p=0.57), vomiting (17.7 versus 16.7%, p=0.95), or tachycardia (14.1 versus 16.7%, p=0.86). Patient demographics were compared between patients with toxic serum digoxin concentrations and those with non-toxic serum digoxin concentrations (Table 1). The female gender was associated with toxic serum digoxin concentrations (p=0.02). Although males had an overall lower serum digoxin concentrations than females, this absolute value did not reach statistical significance (0.94±0.59 versus 1.07±0.79, p=0.38). Additional patient demographics were otherwise similar and not significant between the two groups (Table 1). The presence of digoxin effect on electrocardiogram was not different in patients with elevated serum digoxin concentrations compared with those without (17.7 versus 33.3%, p=0.49). A single patient with evidence of digoxin toxicity on electrocardiogram (bidirectional ventricular tachycardia) did not have toxic serum digoxin concentrations (1.1 ng/ml).
Table 1 Comparison of patients with toxic and non-toxic serum digoxin concentrations.
To evaluate trends in serum electrolyte concentrations with increasing digoxin levels, serum digoxin concentrations were broken down into three categories – >2, 1–2, and<1 ng/ml. Comparison of serum electrolytes at the time of serum digoxin concentration assessment demonstrated a significant difference in magnesium levels between the two groups: patients were more likely to be hypomagnesaemic in the toxic serum digoxin concentrations group compared with the non-toxic serum digoxin concentrations group (p=0.01). Patients with toxic serum digoxin concentrations were also more likely to have hypokalaemia compared with patients with non-toxic serum digoxin concentrations, although this level did not reach statistical significance (Fig 1; Table 2).
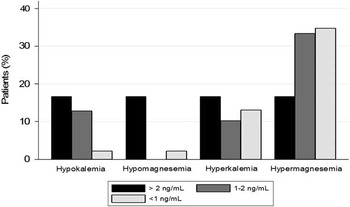
Figure 1 Serum electrolyte concentrations in patients by serum digoxin concentration.
Discussion
Digoxin has been a mainstay in paediatric cardiovascular pharmacotherapy for treatment of arrhythmias and heart failure;Reference Sanatani, Potts and Reed 16 , Reference Rosenthal, Chrisant and Edens 17 however, there are few data guiding the use of serum digoxin concentrations in children, despite widespread digoxin use, particularly for patients with CHD. Although its utility has been challenged, serum digoxin concentrations have traditionally been used to assess digoxin toxicity in adults, and the same practice of following serum digoxin concentrations has been extrapolated for the paediatric population receiving digoxin treatment.Reference Ordog, Benaron, Bhasin, Wasserberger and Balasubramanium 8 , Reference Rutledge 12
Data from our study demonstrate that in paediatric populations receiving digoxin, there is a lack of association between serum digoxin concentrations and signs and symptoms of digoxin toxicity. Thus, the safety of digoxin in this paediatric population cannot be absolutely confirmed based on “non-toxic” or “toxic” serum digoxin concentrations alone. The classic symptoms of digoxin toxicity – for example, nausea, anorexia, vomiting, and visual disturbances/visual halos – were not directly associated with serum digoxin concentrations in our cohort. Similar results demonstrating signs and symptoms of digoxin toxicity in the presence of normal serum digoxin levels in adult and children (2 days to 16 years of age) have also been reported.Reference Ordog, Benaron, Bhasin, Wasserberger and Balasubramanium 8 , Reference Koren and Parker 11 This lack of association between serum digoxin concentrations and signs and symptoms of digoxin toxicity in the paediatric population is likely multifactorial. Reporting of certain symptoms – for example, nausea or visual disturbances – may be limited by language development in the youngest or developmentally delayed patients. Co-morbidities in patients, such as viral gastroenteritis, worsening heart failure, etc., could also account for physical signs and symptoms typically associated with digoxin toxicity, such as anorexia, vomiting, etc., as many of the patients in the cohort had CHD and were being treated for heart failure. Serum digoxin concentrations should only be taken as part of a complete assessment of digoxin toxicity in children.
Electrocardiogram findings in our patient cohort were also not indicative of toxic serum digoxin concentrations. Interestingly, only one patient had an electrocardiogram finding associated with digoxin toxicity (bidirectional ventricular tachycardia), and this patient did not have a toxic serum digoxin concentrations at the time of this arrhythmia. In general, changes in electrocardiogram compared with baseline electrocardiogram (when available) coupled with a history of digoxin use should warrant suspicion of digoxin toxicity.Reference Hastreiter, van der Horst and Chow-Tung 18 On the other hand, concerning electrocardiogram changes can be difficult to interpret, particularly in a patient being treated for an arrhythmia or a patient with a baseline arrhythmia before digoxin treatment initiation. Careful electrocardiogram interpretation and comparison with baseline electrocardiogram should comprise a portion of the evaluation for digoxin toxicity in children receiving digoxin treatment.
Despite the lack of association between serum digoxin concentrations and signs and symptoms of digoxin toxicity, there were a number of significant findings from our analysis. First, only female patients had serum digoxin concentrations in the toxic range (>2 ng/dl). This parallels the adult literature in which studies have identified women to be at higher risk for intoxication in comparison with men.Reference Kanji and MacLean 2 , Reference Yang, Shah and Criley 19 Similarly, in our cohort of patients, females trended towards having higher serum digoxin concentrations, although this result was not statistically significant. This may be due to a unique volume of distribution in female patients. Girls may be more likely to experience symptoms of digoxin toxicity, and future investigations should take into account gender when assessing digoxin dosing and effect in children.
Electrolyte disturbances, in particular hypokalaemia and hypomagnesaemia, are known precipitating factors for signs and symptoms of digoxin toxicity.Reference Hastreiter, van der Horst and Chow-Tung 18 , Reference Bauman, Didomenico and Galanter 20 Not surprisingly, such electrolyte derangements were more commonly noted with increasing serum digoxin concentrations in our study. In particular, there was a statistically significant positive association between decreasing magnesium concentrations and increasing serum digoxin concentrations. This again is similar to findings in the adult literature, which show that patients with toxic serum digoxin concentrations are more likely to be hypomagnesaemic and hypokalaemic.Reference Yang, Shah and Criley 19 Evaluation of serum electrolytes in all patients with suspected digoxin toxicity may be useful as hypomagnesemia and hypokalaemia may cause signs and symptoms of digoxin toxicity even when serum digoxin concentrations are not in the toxic range.Reference Dec 21 We suggest that monitoring serum electrolytes during digoxin therapy may be more useful than monitoring serum digoxin concentrations for preventing digoxin toxicity.
Our study had several limitations. First, this was a retrospective study, which lends itself to relying on others for accurate record-keeping. Second, the number of patients with toxic serum digoxin concentrations was small, and the conclusions to be drawn from this report should be tempered with this mind. Third, the timing of serum digoxin concentrations in relation to the dose of digoxin was not ascertained and may have potentially affected our results. Although the half-life of digoxin is long – 1 to 2 days in patients with normal kidney function – and the sampling likely occurred at steady-state, we cannot absolutely verify the presence of steady-state pharmacokinetics in our patient cohort. Despite these limitations, we were able to meet our objective of assessing the association between toxic serum digoxin concentrations and signs and symptoms of digoxin toxicity in children. Our findings and conclusions are similar to those previously published in the adult literature.
An area outside the scope of this report is the aetiology of digoxin toxicity. Patients may have become toxic due to drug–drug interactions, inter-patient differences in pharmacokinetics, renal insufficiency, or errors in dosing.Reference Hastreiter, van der Horst and Chow-Tung 18 , Reference Koren, Hesslein and MacLeod 22 , Reference Ratnapalan, Griffiths, Costei, Benson and Koren 23 We did not investigate any of these reasons for toxic serum digoxin concentrations. Ascertainment of the cause of patient digoxin toxicity should be of utmost importance. Decreasing the use of digoxin in children is also an option to prevent digoxin toxicity. Although digoxin is frequently used in children for the treatment of heart failure and arrhythmias, its efficacy in heart failure is disputed and alternative and equally efficacious therapies are often available for the treatment of supraventricular arrhythmias.Reference Sanatani, Potts and Reed 16 , Reference Rosenthal, Chrisant and Edens 17
Given the aforementioned reasons, the utility of serum digoxin concentrations for determining digoxin toxicity in children who have signs and symptoms that may be consistent with digoxin toxicity appears to be of limited value. We, therefore, recommend that toxicity be evaluated on a multifactorial basis, incorporating all potential data, when determining the utility of digoxin therapy in a child. In addition, parents and caretakers should receive anticipatory guidance and education regarding both classic and non-classic symptoms associated with digoxin toxicity.
Conclusion
Common signs and symptoms of digoxin toxicity may not be associated with toxic serum digoxin concentrations in children. The utility of serum digoxin concentrations for assessing digoxin toxicity in children requires further evaluation and assessment (Table 2).
Table 2 Comparison of serum potassium and magnesium by serum digoxin concentrations.

Acknowledgement
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the Baylor College of Medicine Institutional Review Board.






