History of the transcatheter approach
Surgical treatment of pulmonary artery stenosis has long been in practice, dating back to before the advent and establishment of simple balloon angioplasty in the early 1980s.Reference Bacha and Kreutzer 1 – Reference Lock, Niemi and Einzig 3 The obvious benefits of increasing diseased vessel diameter, improving pulmonary artery perfusion, and decreasing right ventricular pressure justify the popularity of the technique.Reference Kan, Marvin and Bass 4 Simple angioplasty has continued to be the standard of treatment since its introduction, and provides the cornerstone for initial therapy with low complication rate and ability to treat effectively with minimal risk, as previously reviewed in Bergersen and Lock.Reference Bergersen and Lock 2 The development of improved guidewire platforms, advanced catheter techniques, lower-profile balloons, larger balloons, high-pressure balloons, and cutting balloons has made simple balloon angioplasty the preferred method of treatment for patients with more aggressively diseased vessels.
High-pressure balloons and cutting balloons offer an effective method of treatment for pulmonary artery stenosis, particularly in vessels resistant to low-pressure balloon dilation. As there is still a specific population of lesions that do not respond well to low- or high-pressure balloon angioplasty, cutting-balloon angioplasty has become the next step in therapeutic evolution. This technique uses a balloon with multiple microatherotomes – cutting blades – that are exposed to balloon inflation, allowing the interventionist to strategically make cuts into the lesion to increase the vessel diameter. This is an effective strategy, but they do still hold potential complications, which must be considered.
In the late 1980s, Mullins et al developed a method involving deployment of stents into the diseased vessel to increase the diameter of the vessel, while providing a rigid scaffold to maintain patency.Reference Bergersen and Lock 2 , Reference Mullins, O’Laughlin and Vick 5 Bergersen and LockReference Bergersen and Lock 2 highlighted that stents are particularly effective in the treatment of large and compliant vessels, as well as vessels that might be compressed by adjacent structures. Stenting provides interventionists the technology to provide the patient’s vessel with a rigid framework to avoid compression. Stents still have their limitations, which are reflected in the hesitation to use stents in some lesions such as the ones distal to the pulmonary arterial tree. Furthermore, the populations that are usually treated with pulmonary artery stenosis are children. In addition, during development, children undergo exponential growth of the pulmonary system. This includes vascular branching from the heart, which results in further hesitation when considering stent placement. These disadvantages to the use of existing stent technology provide an opportunity for breakthrough research.
General population involved in pulmonary arterial stenosis
Pulmonary artery stenosis is a congenital stenosis, or narrowing, of vessels from the right side of the heart, which lead to the lungs. Narrowing of the vessel can occur at numerous points throughout the pulmonary arterial tree. The location of the narrowing defines the degree of risk that it, and potential interventions, poses to the patient. Many patients born with CHD, specifically pulmonary artery stenosis, are treated at a young age depending on the severity of the condition and vascular obstruction. In addition, other findings such as elevated right ventricular or main pulmonary artery pressure, unbalanced lung perfusion, and systemic cyanosis may dictate therapy.Reference Feltes, Bacha and Beekman 6
As many high-risk individuals affected by pulmonary artery stenosis are neonates and children, there are many factors to be considered when determining the appropriate intervention to treat the disease. Most of the time, when a patient exhibits severe pulmonary artery stenosis, it is acceptable to intervene. This is because an increased pulmonary gradient compounded with decreased efficiency of circulation of blood from the heart leads to ventricular dysfunction, which may result in pulmonary hypertension and further vascular disease.Reference Bergersen and Lock 2
Even with treatment being paramount, exponential growth in this patient population must be considered. Moreover, it is not merely an increase in size that must be considered, but morphologic change during this time. Dammann and Ferencz articulated this when they described thinning and expanding of pulmonary arteries in the early stages of life.Reference Davies and Reid 7 Future technology must address the dynamic development of the pulmonary arterial system in young patients.
Methods for treatment of pulmonary arterial stenosis
Surgery
Surgical intervention for pulmonary artery stenosis is the oldest treatment modality and predates the oldest modern transcatheter intervention, the simple balloon angioplasty, which was pioneered in 1982.Reference Kan, Marvin and Bass 4 Today, surgical intervention is reserved for more complex diagnoses or in cases in which intervention is not possible. Surgical intervention presents significant procedural risks and uncertain benefits. Trant et alReference Trant, O’Laughlin, Ungerleider and Garson 8 reported branch pulmonary artery stenosis surgical success rates to be only 62%, acutely. The inherent risks associated with surgical intervention, including procedural complications, prolonged hospitalisation and recovery times, and high costs per procedure, make surgery less appealing than alternative therapies.Reference Trant, O’Laughlin, Ungerleider and Garson 8 The favourable risks and safety profiles of catheter-based therapy, when compared with the benefits of a surgical approach, explain why physicians reserve treatment of pulmonary artery stenosis with surgery for situations in which additional anatomic corrections must be made as well.
Balloon angioplasty
Balloon angioplasty was the first intervention of its kind providing interventionists with a novel approach to a variety of diseases. With the advent of this technology, physicians have been able to treat patients with a minimally invasive technique that exhibits reduced recovery time and cost to the patient. Balloon dilations are performed by inflating the device in the narrowed vessel until the elimination of the waist, inferring that the vessel has been successfully expanded. The dismissal of the waist corresponds to the tearing of the intima and media of the vessel wall, or a “therapeutic tear”.Reference Bergersen and Lock 9 Inflation of balloon at high pressures has led to reported success rates of up to 72% in pulmonary angioplasties.Reference Bergersen and Lock 9
Balloon angioplasty inherently contains a variety of positive attributes; however, it does present potential risks that should be considered. Dilation of the vessel with this method can lead to complications including excessive tearing of the arterial wall, hypotension, and aneurysm. Despite present risks, years of practice and effective complication management strategies, such as covered stents and vessel occlusion, have led to reported mortality rates of <1%Reference Bergersen and Lock 9 (Table 1). With complications rates being low, Holzer et alReference Holzer, Beekman and Benson 10 specifically examined cases that underwent balloon angioplasty of the proximal pulmonary artery from 238 patients, concluding a 13.0% (31) adverse event rate with a major adverse events rate of 0.8%. This was further studied by Holzer et alReference Holzer, Gauvreau and Kreutzer 11 in a larger patient population, which concluded an adverse event rate of 22% in 931 patients undergoing balloon angioplasty with an inclusive balloon pressure range. Another aspect inspected was the incidence of adverse events in relation to the age of the patient; balloon angioplasty is often used to treat young children. Researchers found that the highest rate of adverse events occurs in the youngest patients. In addition, the chances of re-stenosis of the treated vessel can range from 10 to 42%. Despite the variable success rate, specifically on resistant pulmonary artery stenosis lesions, the lack of alternative therapeutic options has made balloon angioplasty the favoured method to treat pulmonary artery stenosis.
Table 1 Comparison of adverse events experienced during pulmonary artery stenosis intervention.Reference Gentles, Lock and Perry 13 - Reference Geggel, Gauvreau and Lock 18

PA=pulmonary artery.
Cutting balloons
The introduction of the cutting balloon (Boston Scientific Inc., Natick, Massachusetts, United States of America) 10 years ago made it possible to treat lesions in the pulmonary artery that are resistant to balloon angioplasty alone. The blade feature of the low-pressure balloon allows for a greater precision therapeutic tear of the vessel, which significantly reduces the likelihood of re-stenosis.Reference Bergersen and Lock 9 A multi-centre investigational device exemption study that compared the use of cutting balloons and high-pressure balloon angioplasty for the treatment of resistant pulmonary artery stenosis found that both procedures exhibited nearly the same safety profiles.Reference Bergersen, Justino and Nugent 12 The patients treated with cutting balloon exhibited a 3% rate of adverse events, compared with the high-pressure balloon, which was 2%, with some adverse events possibly correlated to non-device complications. In addition, treatment with a cutting balloon was exponentially more effective in increasing the lumen diameter of the treated vessels when compared with the high-pressure balloons treatment alone (85 versus 52%; p=0.004).Reference Bergersen, Justino and Nugent 12 Figure 1 shows cutting balloons prove to be more effective in addressing some limitations of simple balloon angioplasty, specifically in resistant vessels treated using low- or high-pressure balloon alone. Nevertheless, even through improved techniques, the challenge of treating vessels with obstructions caused by compression, kinking, or recoil has proved challenging with balloon angioplasty alone and remains reliant on stent technology (Fig 1).Reference Bacha and Kreutzer 1
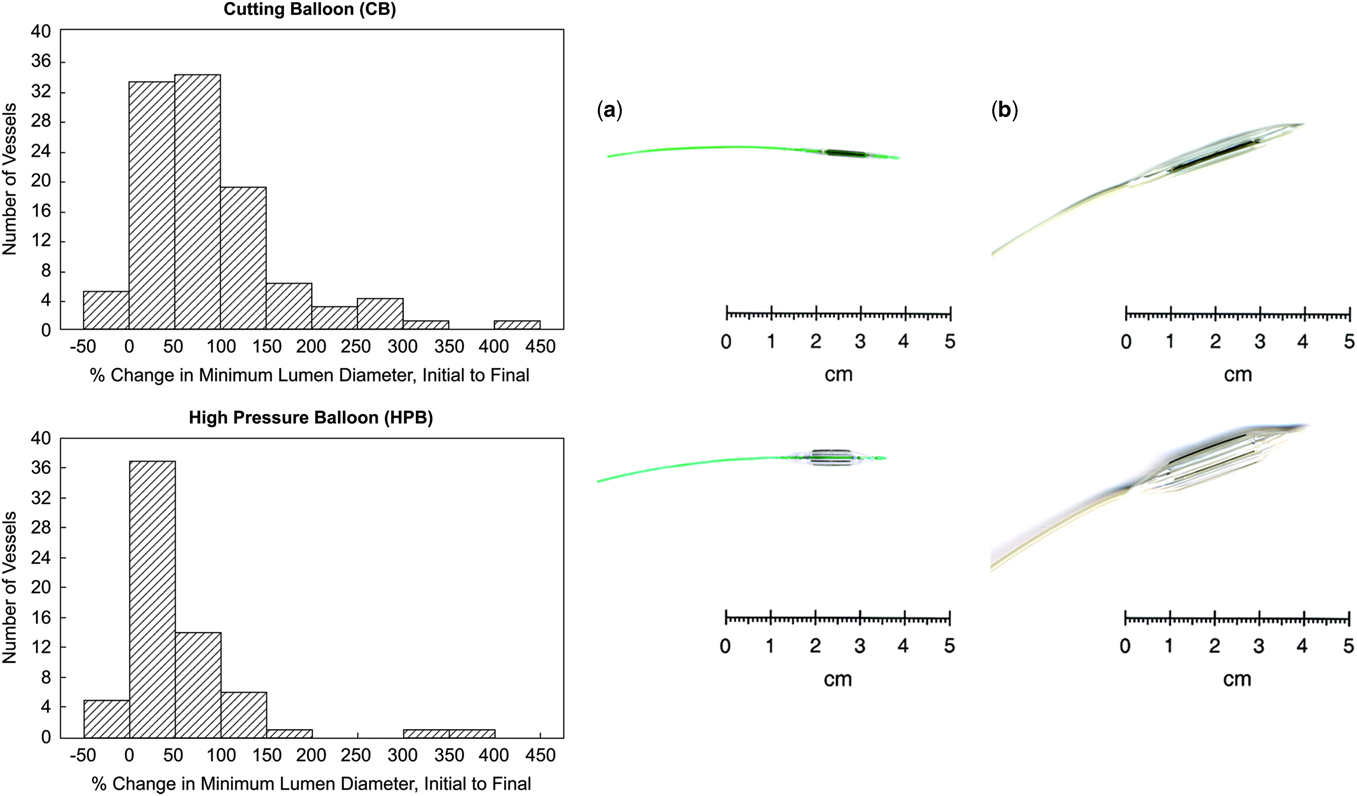
Figure 1 Cutting balloons (CB; Boston Scientific, Inc., Natick, Massachusetts, United States of America) are noncompliant balloons with atherotomes (microsurgical blades) mounted longitudinally on their outer surface. ( a ) The Flextome CB. ( b ) A magnified 2-cm-long peripheral CB device deflated and inflated (Bergersen et alReference Bergersen, Gauvreau and Marshall 21 ).
Bare metal stents
Bare metal stents have advanced the reach of interventional cardiology, allowing physicians to dilate vessels that were unable to be adequately treated with balloon angioplasty alone (Fig 2). They provide a rigid framework for vessels that tend to have recoil characteristics secondary to external pressure and anatomic distortion.Reference Bergersen and Lock 9 This allows for suitable blood flow through the vessel, which can relieve stress on the right side of the heart and increase perfusion to developing lungs. The major risk associated with stents is growth restriction; stents remain at the implanted size as the surrounding vasculature grows with the developing child. Most patients treated for pulmonary artery stenosis undergo additional interventions in their youth to serially dilate implanted stents to accommodate somatic growth (Fig 2).
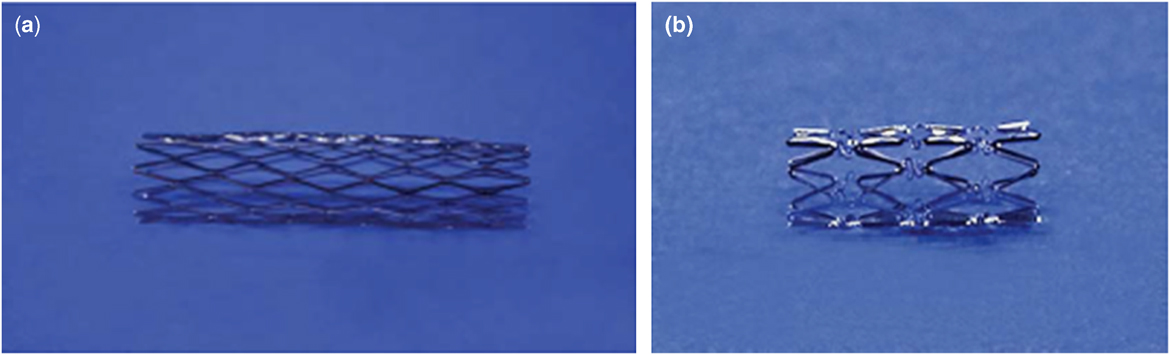
Figure 2 ( a ) Palmaz stent. ( b ) Genesis stent (Bergersen and LockReference Bergersen and Lock 2 ). Common Bare Metal Stents.
Since the introduction of stents, the variety of bare metal stents have increased significantly. They now include different cell configurations, are self- and balloon-expanding, and can be of variable size and shape to further accommodate the location of the lesion. The advancement of bare metal stents design and flexibility, along with the decreased profile within the vessel, allows for them to be placed in diverse locations within the pulmonary tree. A major advantage of many new stents is that they are self-expanding, which decreases the size of the catheter an interventionist must use in order to position the stent and treat the diagnosed vessel. Despite advancements, stent designs are still limited in expansion by re-dilation of the stent. In addition, there still is a possibility of stent fracture and/or loss of integrity and compression from external structures.
The continued development of stent design technology provides a wide breadth of options for treatment of pulmonary artery stenosis; however, the major shortcoming of stent technology is the fixed diameter of the stent in a growing patient. The re-intervention often required to accommodate growth in relation to adjacent vasculature is an aspect that is of great concern in paediatrics, as removal can only be conducted via surgery and expansion can incur risk. Covered stents are used in pulmonary artery stenosis as a bailout technique to avoid emergent surgery in the event of vascular injury, which may compromise the vessel and result in unconfined bleeding into the chest. The development and advancement of stent technology provide a variety of treatment options for interventionists when considering pulmonary artery stenosis; however, gaps in technology are still present. This can be addressed through research and production of a stent that will provide the patient’s vessel with a firm framework while allowing the vessel to grow and proceed with dynamic changes that occur in the early years of life.
In a study conducted in 2013, Takao et al researched the effectiveness of stent placement in young patients. The study investigated the indirect effect of somatic growth in the stented pulmonary artery diameter as compared with the collateral pulmonary artery diameter.Reference Takao, Said, Connolly, Hamzeh and Ing 19 Most patients received a follow-up catheterisation 20+13.5 months after the first stent was placed. They observed that the stent had a decreased diameter of almost 10% at the pulmonary artery, whereas the non-stented collateral exhibited a 16% increase in diameter. Both distal and proximal pulmonary artery vessels from the stent location had similar growth. Comparable results were produced when patients underwent a second follow-up catheterisation months from the initial procedure. Takao et al displayed that stent placement encourages normal distal pulmonary artery growth in patients. Furthermore, the researchers made a critical observation that the younger and smaller – lower body surface area – a patient was at the time of the intervention, the greater the bilateral growth that the patient exhibited in pulmonary vessels.Reference Takao, Said, Connolly, Hamzeh and Ing 19
The study conducted by Takao et alReference Takao, Said, Connolly, Hamzeh and Ing 19 not only demonstrated the benefits of stent placement in younger patients, but also highlighted the prevalence of the re-intervention often required for this patient population. In total, 66% of patients who took part in the study were required to have follow-up intervention to expand the stent that was initially placed. Most bare metal stents that are placed compel physicians to re-intervene later in order to expand the stent to accommodate somatic growth. In select cases, surgical intervention is needed to remove the stent if it is no longer adequate for the vessel diameter.
Stent fractures and safety
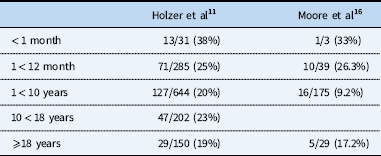
Along with growth concerns, mechanical stressors imparted by the local environment can compromise stent structural longevity. McElhinney et al described the repetitive cyclic stress placed on stents that causes fractures. In the examination of 166 stents, they found that 35 (21%) exhibited complete axial or complex fractures.Reference McElhinney, Bergersen and Marshall 20 The resorting fractures caused haemodynamically significant vessel obstruction in 80% of cases.Reference McElhinney, Bergersen and Marshall 20 Bergersen et alReference Bergersen, Gauvreau and Marshall 21 analysed nearly 4000 cases and determined four procedure-type risk categories that would aid in predicting the risk associated with congenital heart procedures.Reference Bergersen, Gauvreau and Marshall 21 Balloon angioplasty, stent placement, and stent re-dilation were placed in Risk Category 3 or 4, with Risk Category 4 being the highest risk associated with procedures; the odds of a level 4 or 5 adverse event increases exponentially in these specific risk categories. Despite the potential risks, treatment has been proven to be highly effective. In a review conducted by Holzer et al,Reference Holzer, Beekman and Benson 10 a total of 287 total stenoses in 245 patients from eight centres were analysed and exhibited a 99.3% success rate of the procedure. Among the 245 patients, there were 244 adverse events recorded in 32 patients (13.2%), with three (1.2%) being major adverse events. Holzer et alReference Holzer, Beekman and Benson 10 specifically analysed cases that included the balloon and stenting of the proximal pulmonary artery from 48 patients, concluding a 10.4 % adverse event rate. A critical observation across multiple studies shows that there is a decreased adverse event rate as patients age (Table 2).Reference Holzer, Gauvreau and Kreutzer 11 , Reference Moore, Vincent and Beekman 22 Ideally, younger patients could be treated with balloon angioplasty until they are older, whereupon bare metal stents could be placed with less risk and require less invasive procedures for serial stent re-dilation to accommodate for somatic growth. In patients with balloon angioplasty treatment-resistant lesions, advances in stent technology may provide an alternative, and hopefully superior, management strategy.
Table 2 Adverse event (AE) by age: incidence of any AE in patients undergoing pulmonary artery balloon angioplasty and/or stent placement.
Re-intervention
The placement of bare metal stents is considered one of the most effective intervention methods to help the structural integrity of the treated vessel. However, despite short-term success, recent studies show that the rate of long-term effectiveness is far from ideal.Reference Angtuaco, Sachdeva and Jaquiss 23 The rate of re-intervention was previously reported as high at 43%; other reports suggest that intervention for the initial procedure is approximately 11% by 18 months and upwards of 30% by three years.Reference Angtuaco, Sachdeva and Jaquiss 23 , Reference Shaffer, Mullins and Grifka 24
Re-intervention of post-stent placement is most commonly performed to increase the size of the stent as the patient grows. During the first 18 months of the patient’s life, fetal pulmonary arteries grow rapidly, necessitating repeat intervention on a static stent. As technology develops, stent designs have made it possible for re-dilation up to 2 years after the placement of the stent in the vessel – allowing stents to expand with the somatic growth of the patient’s vessel up to a certain point.Reference Bergersen and Lock 9 Ultra-high-pressure balloons have made it possible to expand placed stents with a higher success rate (91%). Maglione et alReference Maglione, Bergersen, Lock and McElhinney 25 reported that the median change exhibited in the vessel diameter was 36%, ranging from 7 to 220% of the original diameter, when the waist of the ultra-high-pressure balloon was eliminated.
Nevertheless, as reported by Bergersen and Lock, stents have limited ultimate expansion diameter. If potential expansion is insufficient, the stent will have to be surgically removed.Reference Bergersen and Lock 9 This dogma has been challenged over the past two decades, initially by “growth” stents and more recently by “unzipping” techniques.Reference Sigler, Schneider and Meissler 26 – Reference Ing, Fagan, Kearny and Mullins 29
Bioresorbable stent
Currently, the development of bioresorbable stent technology is an important advancement for the paediatric population who require stent therapy.Reference Shibbani, Kenny and McElhinney 30 Indeed, even before an ideal purpose-built paediatric device is available, paediatric interventionists have used existing bioresorbable stent for alternate indications in the paediatric population.Reference Zartner 31 – Reference McCrossan 33 Re-intervention of older bare metal stents is usually conducted to increase the size of the stent as the patient grows, or less commonly to treat stent re-stenosis or fracture. Bioresorbable stents could provide a temporary scaffolding to increase vessel size and may obviate the need for re-intervention through serial dilation as they are able to grow with the patient. Research on older bare metal stents has concluded that re-intervention rates may be as high as 43%.Reference Shaffer, Mullins and Grifka 24 Decreasing the amount, or at least the level of intervention needed, may provide substantial direct benefit to the patient.Reference Alexy and Levi 35
Researchers are still investigating the ideal structural integrity of the bioresorbable stent to make it capable to hold up to the pressure within the vascular system. Ideally, the bioresorbable stent is designed to expand vessel stenosis and exhibit intended reabsorption over time. However, to what extent this design will compromise sufficient rigidity of the framework, and thus vessel lumen, remains to be seen. This programmed loss of structural integrity permits the patient’s body to have time to heal the vessel, and allow continued vessel growth with the normal growth of the body.Reference Feltes, Bacha and Beekman 6 , Reference Alexy and Levi 35 During this gradual reabsorption, the foundational structural scaffold of the stent is still within the vessel, which provides the vessel the integral support it needs to prevent re-stenosis.Reference Alexy and Levi 35 If further intervention is needed, interventionists can delay additional therapy until both the vessel and patient have grown and can accommodate larger available technology. This would avoid the conundrum of using permanent bare metal stents that cannot grow to adult size, even with balloon dilation, and may necessitate surgical removal from smaller-diameter vessels.
It seems reasonable to expect that the potential risks associated with the bioresorbable stent will be on par with other interventional methods to treat pulmonary artery stenosis. Bioresorbable stent technology resembles the previous and currently used stents to a great degree. As bioresorbable stents have inherited many characteristics from previous stents, it is very reasonable to assume that they will exhibit similar safety concerns. This is not to say that it might display risks unique to the bioresorbable characteristics of the stent. Bioresorbable stent technology risks could include inflammation-based constrictive remodelling, vascular biocompatibility, long-term stent failure, and in-stent stenosis.
Bioresorbable stenting is not a novel idea. Many physicians have used bioresorbable stents, which were designed for other uses, to help patients through various pulmonary artery stenosis. ZartnerReference Zartner 31 published a case where a magnesium stent was used in a female preterm baby born at the 26th week of gestation. In addition, in a case presented by Rodriguez et al a bioresorbable vascular scaffold, which is usually reserved for coronary intervention, was used to treat a 3-month-old child. They cited the off-label use of the device as it would allow for them to open the occluded vessel, while taking into account the growth of the pulmonary circulation.Reference Castro Rodriguez 32 Many positive aspects of bioresorbable stents can be seen in these cases (Fig 3).Reference Zartner 31 – Reference McCrossan 33
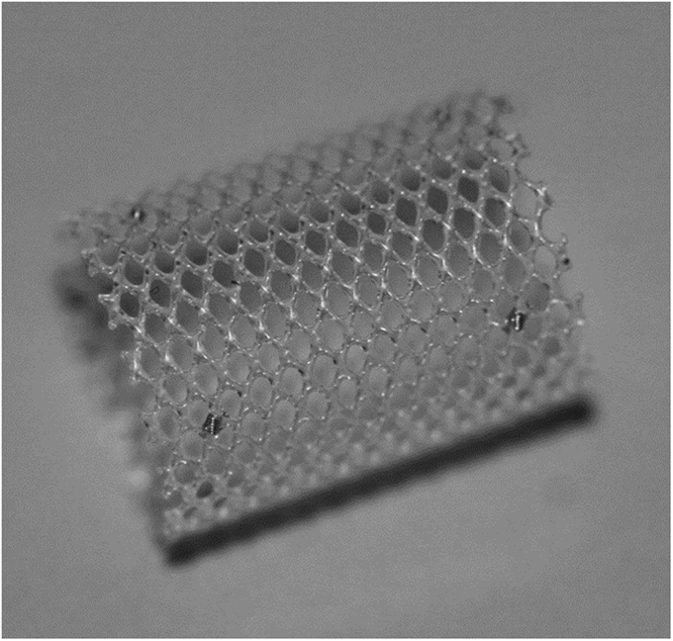
Figure 3 480 Biomedical Inc. bioresorbable stent. 34
Currently, the paediatric bioresorbable stent closest to moving forward with a clinical trial is in the final phases of development and is being manufactured by 480 Biomedical Stent Inc. The stent and delivery system have been specifically designed for application in children with pulmonary artery stenosis. Unlike many technologies in paediatric interventional cardiology that were developed for adult diseases, including present balloon and stent technology, this will be a specific treatment for CHD in the growing child. Support from the National Institutes of Health (National Heart, Lung, and Blood Institute) was contracted to the sponsor after a call for submissions to bring this technology to the CHD population.Reference Core 36 In this context, over the past four years, 480 Biomedical has partnered with the paediatric interventional community to design technology to meet the specifications required to treat children with pulmonary artery stenosis.
Having completed the technology development, the sponsor is in the final phases of proposing an early feasibility clinical trial to the Food and Drug Administration. The purpose-built paediatric bioresorbable stent is designed to support pulmonary artery anatomy affected by pulmonary artery stenosis for a minimum of 3 months, and then relieve its own structural integrity to conform to the somatic growth of the surrounding vessel over the next 6 months. With this unique ability, physicians would not have to intervene surgically to remove metal stents, but have the opportunity, if required, to re-dilate the growing vessel. Bioresorbable stents may ultimately replace the traditional methods of treatment and provide physicians a method of treatment that confidently treats pulmonary artery stenosis patient now, and as the patient grows. The first application of the stent hopes to be in the more proximal vessels, which allows for easier delivery and placement of the stent. With delivery methods suitable for children, the 480 Biomedical bioresorbable stent will be able to provide a much-needed innovative therapy for this specific population of pulmonary artery stenosis patients and could address the issue of growth in small patients.
Despite perceived risks, bioresorbable stents are an essential innovation necessary to breakthrough many of the limitations that hinder the progression of pulmonary artery stenosis care in the paediatric population. These stents will hopefully mitigate the risks that patients currently face throughout their care. They create an intervention that encompasses the cost-effective and decreased hospitalisation benefits of balloon angioplasty and stents, while also providing a safe, long-term solution. Bioresorbable stents will offer vessels the structural support of traditional stents, but will be able to grow with the patient’s vasculature, and rapid somatic growth in the early years of life.
Summary
Even with the continuing improvement of current interventional technology involving balloon angioplasty, cutting balloons, and bare metal stenting of pulmonary artery stenosis, innovation needs to be made. The limitations that have been observed in current standards of practice have not only left a large population of patients unable to be effectively treated but have pushed physicians and researchers to elucidate what is needed to provide the best long-term care of patients with pulmonary artery stenosis. Surgery, balloon angioplasty, and cutting balloons have long been used to effectively increase the size of vessels, resolve pressure gradients, and relieve immediate danger for patients. Further, the advent of bare metal stents allowed interventionists the ability to place devices that would give diseased vessels a structural backbone so that they could be dilated more easily. However, with this rather effective method of treatment, patient growth has forced physicians to dilate the stents via further catheter interventions as much as possible or ultimately surgically remove the implanted stents. The promise of bioresorbable stents possibly holds the solution for many of the issues that plague physicians today when it comes to the treatment of pulmonary artery stenosis in this population. Bioresorbable stents are designed to initially provide a rigid framework for the vessel as it is dilated, allowing the vessel to grow. Thereafter, the stent will intentionally degrade, losing its integrity and permitting normal growth of the vessel with surrounding vasculature. Even though many of the benefits of this groundbreaking technology are evident, risks and uncertainties still exist, which require exploration in clinical trials. The innovation of bioresorbable stents as a treatment for pulmonary artery stenosis holds much hope as the key to a more effective intervention for young patients.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflicts of Interest
L.B. is a Consultant for 480 Biomedical Inc.







