Left pulmonary artery sling is a rare congenital abnormality in which the left pulmonary artery arises from the right pulmonary artery and then passes posteriorly between the trachea and oesophagus to the left pulmonary hilum. It is frequently associated with tracheobronchial abnormalities, congenital heart disease, and lung abnormalities.Reference Gikonyo, Jue and Edwards 1 , Reference Chen, Lee and Lin 2 However, the combined finding of left pulmonary artery sling with right pulmonary hypoplasia and total anomalous pulmonary venous connection is rare; only one case, in which the patient underwent termination of pregnancy prior to 23 weeks of gestation, has been reported in the literature.Reference Hazelzet, Patrier and Barre 3 We present the findings on prenatal ultrasound imaging and their correlation with postnatal computed tomography (CT) imaging in a fetus with these associations.
Case report
A 37-year-old Japanese woman was referred to our hospital at 28 weeks of gestation for evaluation of the abnormal location of the fetal heart on the right side. Fetal echocardiography showed dextrocardia, an enlarged right atrium, a left superior caval vein, and a small right lung (Fig 1a). All four pulmonary veins formed a confluence behind the left atrium (Fig 1b). The confluent vein connected via a vertical vein to the left superior caval vein. Additionally, the confluent vein drained into the left superior caval vein at an increased flow velocity of 0.7 m/second, suggesting pulmonary venous obstruction. The association between total anomalous pulmonary venous connection and right lung hypoplasia is commonly or often seen in scimitar syndromeReference Bhide, Murphy, Thilaganathan and Carvalho 4 ; however, the right pulmonary vein did not drain into the inferior caval vein, and the systemic arterial supply to the right lung was normal. Therefore, the initial diagnosis was total anomalous pulmonary venous connection with pulmonary venous obstruction, persisting left superior caval vein, and right pulmonary hypoplasia.
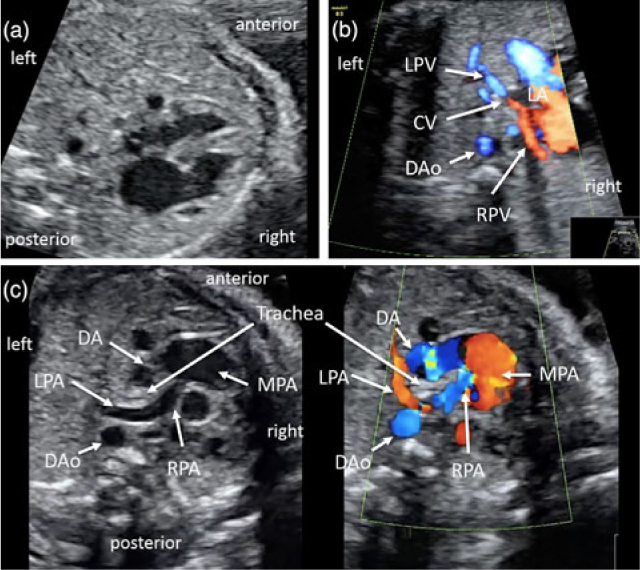
Figure 1. Fetal echocardiography at 28 (a, b) and 30 weeks (c) of gestation. (a) Four-chamber view showing abnormal rotation of the heart towards the right side. (b) Colour Doppler image in an axial view showing that both the left (LPV) and right (RPV) pulmonary veins drained into a confluent vein (CV) posterior to the left atrium (LA) and anterior to the descending aorta (DAo). (c) Grey-scale (left) and colour Doppler (right) images in an axial view showing the left pulmonary artery (LPA) arising distally from the right pulmonary artery (RPA). The LPA turned sharply around the right side of the trachea. Note that the trachea was surrounded by the LPA on the right side and by the ductus arteriosus (DA) or aortic arch on the left side. MPA, main pulmonary artery.
At 39 weeks of gestation, a female infant was delivered by caesarean section due to fetal distress. She weighed 2346 g, and Apgar scores were 3 and 6 at 1 and 5 minutes after birth, respectively. She was transferred to the neonatal intensive care unit due to cardiopulmonary compromise, and ventilation with synchronized intermittent mandatory ventilation was started. Neonatal echocardiography and chest CT confirmed the prenatal diagnosis of total anomalous pulmonary venous connection with pulmonary venous obstruction and left superior caval vein (Fig 2a, b). Additionally, the main pulmonary artery was enlarged and did not bifurcate in the usual location. The right pulmonary artery arose from the right side of the main pulmonary artery. From the left side of the main pulmonary artery, the ductus arteriosus arose and connected to the descending aorta. The left pulmonary artery arose distally from the right pulmonary artery and turned sharply round the right side of the trachea, continuing between the trachea and oesophagus toward the left lung (Fig 2c, d). The left pulmonary artery slightly compressed the main trachea; however, the right lung was hypoplastic and the right pulmonary artery was smaller than the left pulmonary artery. The infant was diagnosed as having left pulmonary artery sling with right pulmonary hypoplasia. The small left atrium and the giant coronary sinus made the anastomosis between the confluent pulmonary vein and the left atrium unfeasible. Therefore, a repair of the anomalous venous return was avoided, and trans-catheter stenting of the stenotic vertical vein, as a bridge to corrective surgery, was carried out. Symptoms of cardiac failure gradually progressed over time. At 8 months of age, the infant died of cardiac failure.

Figure 2. Neonatal CT at birth. (a) Chest CT in an axial view showing that both the left (LPV) and right (RPV) pulmonary veins drained into a confluent vein (CV) posterior to the left atrium (LA) and anterior to the descending aorta (DAo). (b) Volume-rendered image viewed from behind showing a confluent vein (CV) and the vertical vein (VV) draining into the left superior caval vein (LSCV). (c) Chest CT in an axial plane showing the left pulmonary artery (LPA) and ductus arteriosus (DA) encircling the trachea. The right lung is hypoplastic and the right pulmonary artery (RPA) is smaller than the LPA. (d) Volume-rendered image viewed from behind showing an aberrant distal origin of the LPA. The trachea is apparently surrounded by the LPA and DA. Ao, aortic.
Discussion
To date, reports of prenatal ultrasonographic diagnosis of left pulmonary artery sling are limited.Reference Yorioka, Kasamatsu, Kanzaki, Kawataki and Yoo 5 – Reference Li, Li, Saul and Zhao 7 This is likely due to the rarity of this condition and previously limited capability of imaging technology for prenatal detection, because pulmonary artery branches are not routinely investigated during fetal ultrasound examination. In our case, left pulmonary artery sling was identified on the retrospective review of fetal sonographic movie; the left pulmonary artery, arising from the right pulmonary artery, turned sharply round the right side of the trachea, and the ductus arteriosus connected the top of the main pulmonary artery and the descending aorta on the left side of the trachea (Fig 1c).
Major congenital heart diseases have been found to be associated with the incidence of 30–85% in infants with left pulmonary artery sling.Reference Gikonyo, Jue and Edwards 1 , Reference Xie, Juan and Wang 8 These were represented by ventricular septal defect, atrial septal defect, patent ductus arteriosus, persistent left superior caval vein, tetralogy of Fallot, common ventricle, and coarctation of the aorta. However, the association of left pulmonary artery sling with total anomalous pulmonary venous connection is rare; only one case has been reported in the literature.Reference Hazelzet, Patrier and Barre 3 Right lung hypoplasia has also been found to be associated with the incidence of 22% in left pulmonary artery sling.Reference Chen, Lee and Lin 2 Tracheobronchial stenosis has been described in 50–100% of the patients with left pulmonary artery sling.Reference Chen, Lee and Lin 2 , Reference Monnier and Monnier 9 Associated tracheobronchial stenosis frequently requires early surgical intervention and has a poor prognosis due to severe airway obstruction after birth.Reference Gikonyo, Jue and Edwards 1 Therefore, left pulmonary artery sling should be prenatally diagnosed to prepare for surgical intervention for tracheobronchial stenosis.
The aetiology of left pulmonary artery sling remains unknown. Only a few reports have described the association of left pulmonary artery sling with chromosomal abnormalities, such as trisomy 18Reference Derbent, Saygili, Tokel and Baltaci 10 and 21.Reference Alsaied, Sticka, Unaka, Cooper and Manning 11 Recently, Cano Sierra et al.Reference Cano Sierra, Mestra and Ronderos Dumit 12 reported that pulmonary arterial sling is significantly more frequent in patients with Mowat–Wilson syndrome than in the general population. Mowat–Wilson syndrome, a multiple congenital anomaly/mental retardation syndrome, is a genetic condition due to a mutation in the Zinc finger E-box-binding homeobox 2 (ZEB2) gene, previously called ZFHX1B (SIP1) on chromosome 2.Reference Strenge, Heinritz and Zweier 13 However, the molecular mechanisms producing left pulmonary artery sling remain undetermined. Pu et al.Reference Pu, Chung, Hoffer, Jonas and Geva 14 hypothesized that left pulmonary artery sling occurs when the left postbranchial pulmonary arterial vessels cannot connect with the left sixth branchial arch, and a secondary connection is made to the right sixth branchial arch through the embryonic peritracheal primitive mesenchymal vessels.
The prognosis of left pulmonary artery sling is variable depending on clinical presentation. Gikonyo et al.Reference Gikonyo, Jue and Edwards 1 reported that left pulmonary artery sling usually causes airway obstruction before 1 year of age in approximately 90% of cases; however, 14 (12%) of the 130 patients surveyed were asymptomatic. Surgery is the standard of care for all symptomatic patients, as medically managed patients have a death rate of 90%.Reference Marmon, Bye, Haas, Balsara and Dunn 15 In contrast to symptomatic patients, the prognosis for asymptomatic patients is excellent, and surgical intervention is not indicated. In our case, although the left pulmonary artery sling slightly compressed the main trachea and was associated with right pulmonary hypoplasia, the neonate had no respiratory symptoms and complications. On the contrary, the neonate had a total anomalous pulmonary venous connection with pulmonary venous obstruction. Therefore, we prioritized the treatment of the pulmonary venous obstruction over the left pulmonary artery sling. Although the outcomes after surgical correction of total anomalous pulmonary venous connection have generally improved over the past several decades with advances in surgical techniques and medical management, surgical repair remains challenging, with early mortality rates reported in the literature ranging from <10 to 20%.Reference Yoshimura, Fukahara and Yamashita 16 Repair during the neonatal period, the presence of pulmonary venous obstruction, mixed or infracardiac total anomalous pulmonary venous connection, a small left atrium, and low body weight at the time of operation are associated with a higher mortality rate.Reference Yoshimura, Fukahara and Yamashita 16 Our case had almost all of these risk factors: neonate, pulmonary venous obstruction, a small left atrium, and a lower body weight. In addition to the small left atrium, the giant coronary sinus made the anastomosis between the confluent pulmonary vein and the left atrium unfeasible. Recently, stent implantation for the obstruction of venous drainage to delay the timing of surgical correction has been applied to patients considered difficult to surgically repair in the neonatal period.Reference Kitano, Yazaki, Kagisaki and Kurosaki 17 Trans-catheter stenting of the stenotic vertical vein, as a bridge to corrective surgery, was therefore carried out. Although the postoperative state was stable for blood circulation, symptoms of cardiac failure gradually progressed over time. Unfortunately, the neonate died of cardiac failure at 8 months of age.
Our case report indicates that awareness of these rare associations will help avoid prenatal misdiagnosis of left pulmonary artery sling and facilitates the selection and formulation of appropriate treatment strategies for neonates, thus highlighting the importance of the determination of pulmonary artery arrangement by fetal echocardiography if right pulmonary hypoplasia and/or congenital heart disease is suspected.
Author ORCIDs
Kiyomi Tsukimori 0000-0002-5196-2678
Acknowledgements
None.
Financial Support
This case report received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committee of Fukuoka Children’s Hospital. Informed consent was obtained from the patient in this study.




