Descriptive text
A 21-month-old previously healthy girl was admitted to the paediatric cardiac ICU with 3 days of dyspnoea, fussiness, and refusal to eat; she had a 2-week history of upper respiratory infection symptoms. Evaluation in the cardiac ICU noted a dilated heart with severely decreased function.
Admission vitals were as follows: temperature 98.2°F (36.8°C), pulse 134, blood pressure 102/66, respirations 60, and oxygen saturation 99% on room air. Examination was significant for respiratory distress with intercostal retractions and nasal flaring, a regular rhythm with an S3 gallop but no murmurs or rubs, 2+ pulses in all limbs, and severe hepatomegaly with liver edge at the anterior superior iliac spine. Arterial and plethysmograph wave-forms were consistent with pulsus alternans (Fig 1).
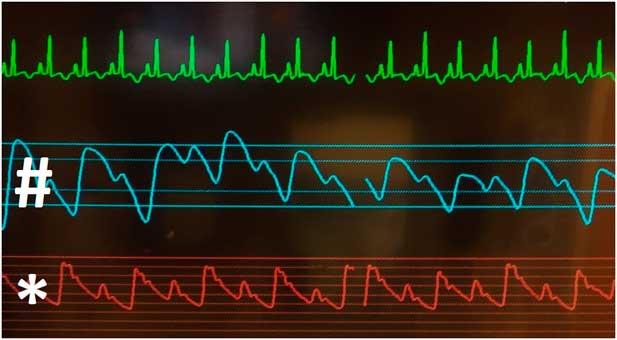
Figure 1 Pulsus alternans demonstrated on cardiopulmonary monitoring. Note the alternating high- and low-amplitude arterial pressure (*) and pulse-oximeter (#) tracings with normal sinus rhythm, and no electrical alternans, on corresponding telemetry.
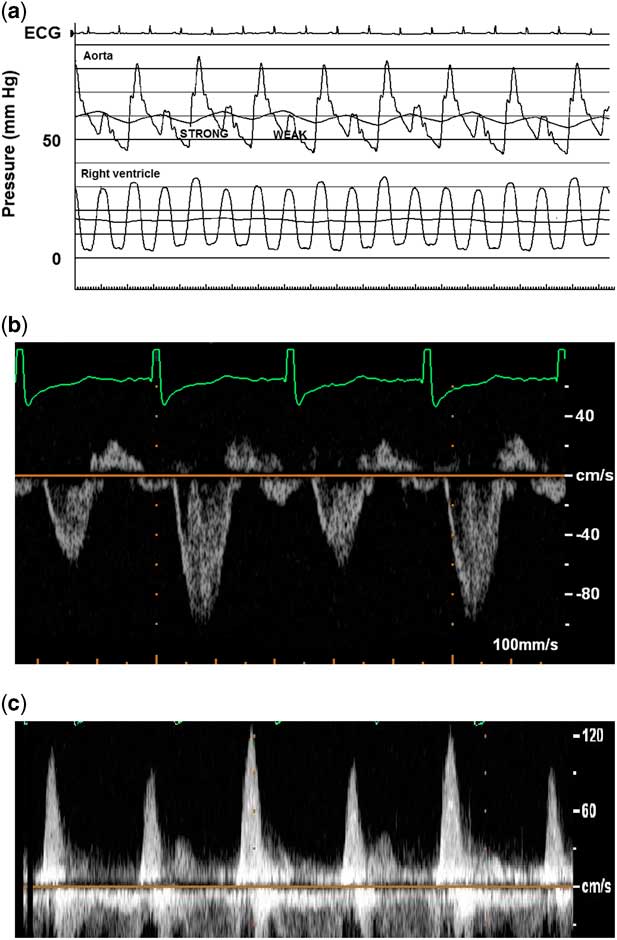
Laboratory analyses showed leucocytosis of 23.6K with a slight neutrophil predominance, troponin I 0.13 ng/mL (reference range<0.09), troponin T 0.06 ng/mL (reference range<0.03), normal creatine kinase MB isoenzyme, and brain natriuretic peptide 48,565 pg/mL (reference range 5–125). Chest X-ray showed an enlarged heart with perihilar and interstitial oedema. Electrocardiogram was significant for sinus tachycardia, right atrial enlargement, and non-specific ST-segment and T-wave changes. Echocardiogram demonstrated markedly decreased left ventricular function (ejection fraction 12%), severe left heart dilation, mild mitral regurgitation, normal coronary origins, and a large thrombus adherent to the left ventricular free wall extending towards the mitral valve apparatus. Right ventricular function was only mildly depressed, significantly less affected than the left heart. Doppler interrogation showed beat-to-beat variation in pulse wave amplitude in the absence of electrical alternans or extrasystolic beats (Fig 2).
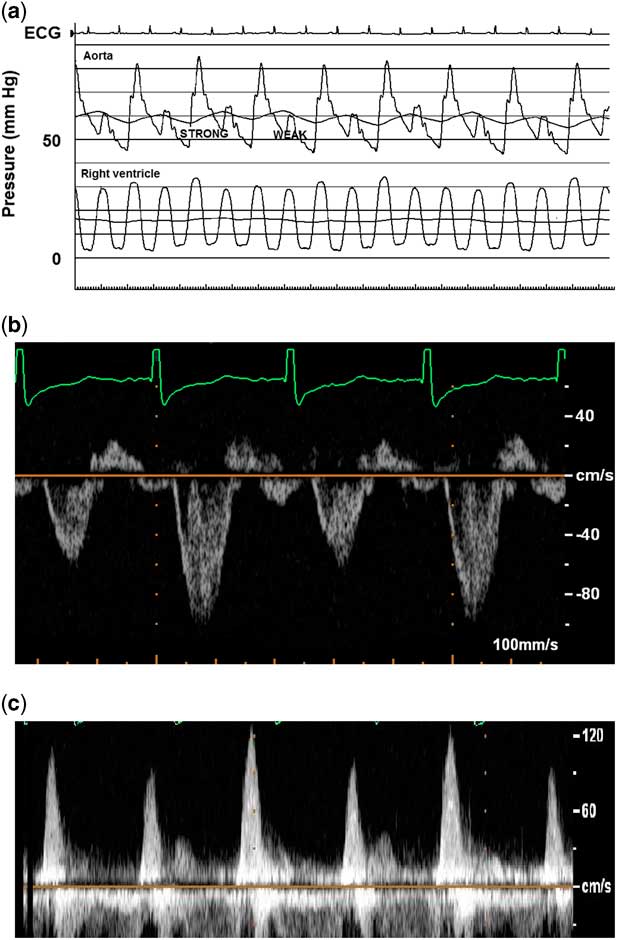
Figure 2 Beat-to-beat alternation in pulse amplitude of aortic pressure tracing ( a ), aortic Doppler interrogation ( b ), and mitral inflow ( c ). Corresponding echocardiogram (ECG) rhythm is sinus, without electrical alternans or extrasystolic beats. Note the lack of pulsus alternans in the right ventricle, although there is respirophasic variation, suggesting that left ventricle pre-load conditions may be involved. Also, there is E and A wave fusion in mitral valve inflow pattern, in the presence of severe diastolic dysfunction.
The patient was started on a milrinone infusion, diuretics, nasal cannula oxygen, and anticoagulation for the thrombus. Owing to persistent cardiac dysfunction, cardiac catheterisation was performed as part of a pre-transplant work-up. Those data were significant for a normal cardiac index of 3 L/minute per m2, increased left ventricular end-diastolic pressure of 14 mmHg, mildly elevated pulmonary vascular resistance of 2.7 indexed Wood units, normal right heart pressures, and normal coronary angiography. Pulsus alternans was again noted, with beat-to-beat alternation in aortic pressure wave amplitude (Fig 2). No alternation was noted with the pulmonary artery wave amplitude, although slight respiratory variation was noted in right ventricle (RV) pressure.
Her thrombus extended and her ventricular function remained poor, so she underwent an operation to evacuate the thrombus and place a ventricular assist device. The assist device was explanted 1 week later and she was initially managed medically. She later underwent cardiac transplantation and is now doing well.
Discussion
Pulsus alternans is a rare phenomenon of alternating strong and weak pulses that occurs with severe ventricular dysfunction. Although it can be seen in association with electrical alternans, it is traditionally defined as mechanical alternans in the presence of a regular rhythm.Reference Harris, Nghiem, Schreiber and Wallace 1 Its presence generally portends a poor prognosis, and recognition by clinical staff is key as it may be the first clinical sign of severe myocardial dysfunction.Reference Weber 2 First described by Traube in 1872, most reported cases are in adults, with few reports in children with congenital aortic stenosis or cardiomyopathy. We have described a case of pulsus alternans in a young girl with severe biventricular dysfunction. The underlying mechanisms are incompletely understood, with theorised aetiologies proposing beat-to-beat changes in left ventricular loading conditions, variations in myocardial oxygen supply/demand, and alternations in myocardial contractility.
Most scholars agree that this phenomenon centres around alternations in left ventricular systolic pressure, but there are inconsistent data relating to potentially predisposing ventricular loading conditions. An initial theory implicated beat-to-beat changes in left ventricular end-diastolic volume and pressure with resultant shifts in Frank–Starling forces as the cause of alternans. More recent series have shown that volume loading conditions play a role but do not explain the entire effect. For example, a series of five children found that left ventricular end-diastolic volume was increased in all beats, presumably secondary to generalised dysfunction, with a small but significant increase in left ventricular end-diastolic volume and pressure preceding the stronger pulsations.Reference Harris, Nghiem, Schreiber and Wallace 1 In those patients, continuous pacing equalised the R-R interval, diminished alterations in the diastolic filling period, and simultaneously decreased the amplitude of the pulsus alternans, suggesting that changes in loading conditions played a central role.Reference Harris, Nghiem, Schreiber and Wallace 1 In fact, we noted respirophasic variation in the RV pressure, which may support this hypothesis (Fig 2a). However, other studies demonstrated no changes in left ventricular volume loading conditions in the majority of patients.Reference Cooper, Braunwald and Morrow 3 , Reference Cohn, Sandler and Hancock 4 A more recent case by Edwards and Cohen demonstrated pulsus alternans without alternation in mitral inflow, further suggesting that haemodynamic factors are not necessary or sufficient causes.Reference Edwards and Cohen 5 Another study of 12 patients with severe aortic stenosis did not identify any changes in left ventricular end-diastolic volume, pressure, or other markers of left ventricular relaxation between the alternating beats, suggesting that loading conditions and Frank–Starling forces are not the primary driving forces.Reference Hess, Surber, Ritter and Krayenbuehl 6
Variations in myocardial oxygen supply/demand have been implicated as well. Cooper et alReference Cooper, Braunwald and Morrow 3 noted that pulsus alternans was associated with an increase in the product of left ventricular systolic pressure and heart rate, as well as a higher aortic valve gradient. Left ventricular systolic pressure-heart rate product was used as a surrogate for cardiac tension–time index, which correlates with cardiac oxygen consumption. A higher aortic valve gradient is associated with decreased coronary oxygen delivery, both by decreasing coronary perfusion pressure and increasing the duration of systole. They theorised that this mismatch between cardiac oxygen supply and demand was an integral component of pulsus alternans. Along these lines, pulsus alternans has been demonstrated in other states of increased oxygen demand (e.g. hypertension) or decreased oxygen delivery (e.g. atherosclerotic coronary disease).Reference Surawicz and Fisch 7 However, the absence of pulsus alternans in congenital aortic stenosis lesions, which also have an increased left ventricular systolic pressure-heart rate product, suggests that altered myocardial oxygen supply/demand is not the primary factor.
Mechanisms that alter left ventricular contractility, resulting in beat-to-beat variations in myocardial contraction, may also underlie pulsus alternans. These mechanisms may be influenced by myocyte contractile force and/or number of recruited myocardial fibres. This is consistent with theories noting alterations in parameters of left ventricular contractility, such as maximum rate of left ventricular pressure rise, and left ventricular shortening/ejection fractions between strong and weak beats.Reference Harris, Nghiem, Schreiber and Wallace 1 , Reference Hess, Surber, Ritter and Krayenbuehl 6 It is also consistent with theories implicating myocardial oxygen supply/demand imbalances.Reference Cooper, Braunwald and Morrow 3 In addition, numerous in vitro and molecular experiments have described changes in cellular calcium uptake and release associated with both electrical and mechanical alternans.Reference Surawicz and Fisch 7 Interestingly, pulsus alternans was noted to recur after exercise in one patient, supporting the postulate that myocardial oxygen supply/demand and contractile forces may play roles together.Reference Harris, Nghiem, Schreiber and Wallace 1 In addition, work by Mitchell noted that weaker beats were associated with shorter end-diastolic myocardial fibre length, regardless of left ventricular end-diastolic pressure, suggesting that alternans develops from an interplay between loading conditions, ventricular compliance, and contractility changes.Reference Mitchell, Sarnoff and Sonnenblick 8
In summary, pulsus alternans is a rare phenomenon seen in multiple pathophysiologic states, often with severe ventricular dysfunction. The phenomenon was initially thought to be secondary to alternations in ventricular loading conditions and altered Frank–Starling forces. However, various reports suggest that it probably has a multifactorial aetiology related to beat-to-beat changes in left ventricular contractility, altered myocardial oxygen supply/demand, and myocyte calcium cycling. Our case underscores the clinical import of pulsus alternans in a critically ill child with dilated cardiomyopathy. It also demonstrates unique echocardiographic findings, which we believe adds to the relatively small pool of data that is currently available regarding pulsus alternans pathophysiology and further suggests that there may be variations in the underlying mechanisms depending on the clinical situation.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The institutional committees of the University of Mississippi Medical Center do not require review of single patient reports.