Case presentation
A 31-year-old woman was referred to our perinatal centre at 21 weeks of gestation for fetal echocardiography, which showed usual atrial situs, concordant atrioventricular connection with discordant ventriculoarterial connection (D-transposition of the great arteries) and pulmonary atresia (Fig 1a and Supplementary video S1). The right and left ventricular sizes were balanced. The left atrium was dilated with severe mitral regurgitation (Fig 1b), and the presence of a non-restrictive left-to-right shunt through the patent foramen ovale was noted. At 22 weeks of gestation, fetal echocardiography revealed a slightly smallish left ventricle, associated with myocardial thickening and an echogenic endocardium, suggesting endocardial fibroelastosis. Fetal growth was normal.
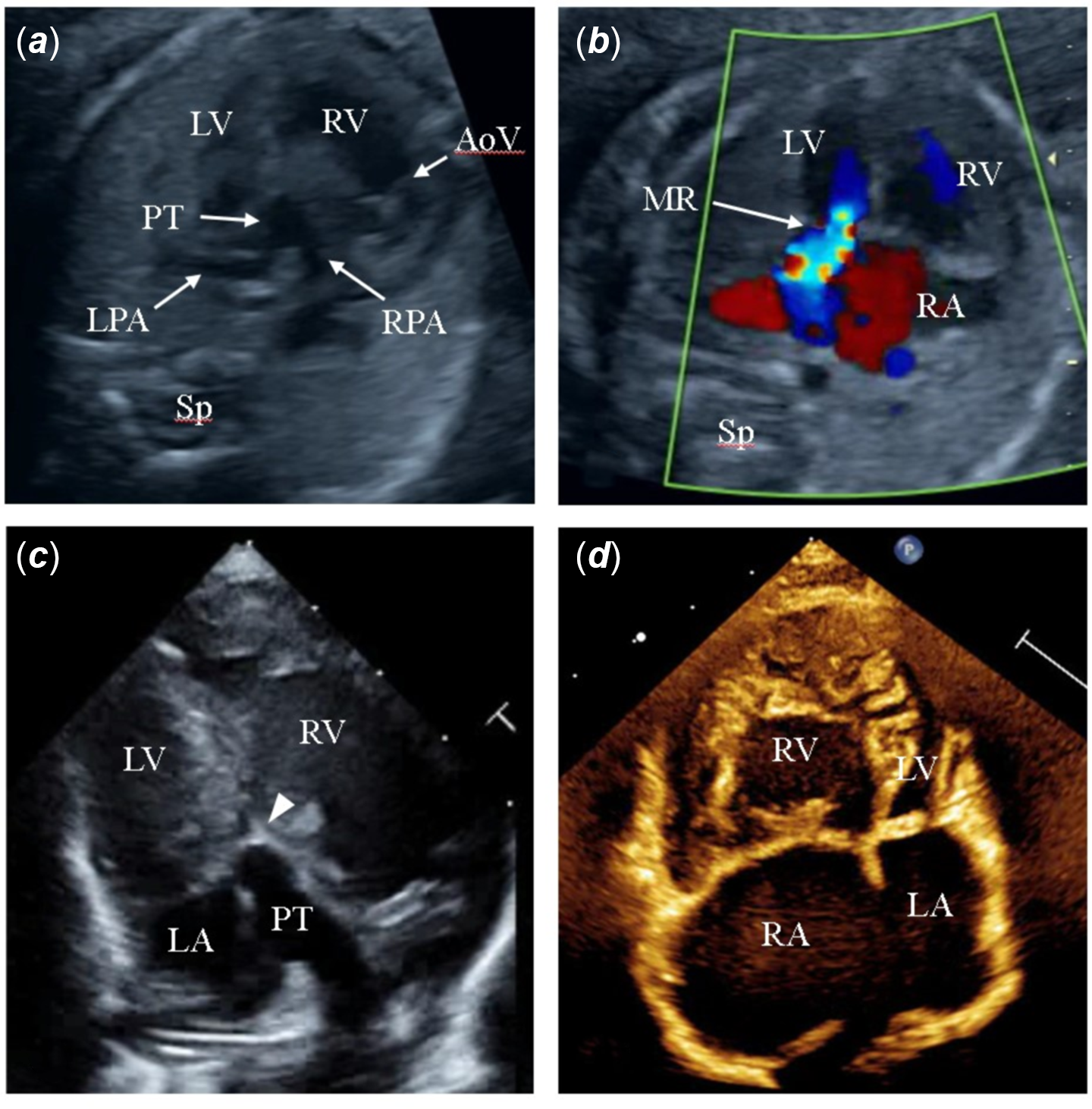
Figure 1. Prenatal echocardiograms at 21 weeks ( a , b ), postnatal transthoracic echocardiogram at birth ( c ) and before the Glenn procedure ( d ). ( a ), Aorta arising from the right ventricle and pulmonary artery arising from the left ventricle. ( b ), Four-chamber view showing severe mitral regurgitation. ( c ), Apical long-axis view shows a hypoplastic left ventricle. The arrowhead shows an imperforate pulmonary valve. ( d ), Apical four-chamber view shows a crescent-shaped deformed left ventricle. aAo = ascending aorta; AoV = Aortic valve; LA = left atrium; LPA = left pulmonary artery; LV = left ventricle; MR = mitral regurgitation; PT = pulmonary trunk; RA = right atrium; RPA = right pulmonary artery; RV = right ventricle; Sp = spine.
The neonate weighed 2.9 kg and was born at 39 weeks gestation. Postnatal echocardiography showed short segment membranous pulmonary atresia and confirmed the prenatal diagnosis (Fig 1c and Supplementary video S2). However, the left ventricle was smaller in size than that during the fetal period. The myocardium was thickened, and the endocardium was brighter. The end-diastolic diameter was 10.3 mm (z score = −5.3), whereas the mitral valve annulus diameter was 11.8 mm (z score = 0.3). Moderate mitral regurgitation with peak flow velocity of 5.0 m/s was observed. The patient had an isolated cleft of the anterior mitral valve leaflet. No coronary artery anomaly was observed.
The patient received prostaglandin E1 infusion to maintain the ductus arteriosus. Mitral regurgitation worsened due to increased pulmonary blood flow. Tachypnea, cardiomegaly, and high levels of B-type natriuretic peptides (1378 pg/mL at 18 days of age) were observed. The patient was treated with sedation and inotropic agents for heart failure.
A left modified Blalock-Taussig shunt (3.5 mm polytetrafluoroethylene graft), partial closure of the mitral valve, and atrial septal defect enlargement were performed at 25 days of age. Intra-operative findings showed that usual atrial situs, D-transposition of great arteries (Fig 2a), and lumen of the main pulmonary trunk were preserved (Fig 2b and c). An expanded polytetrafluoroethylene patch with a 4.0-mm hole was sutured to the mitral annulus to close the mitral valve. Post-operative echocardiography revealed moderate mitral regurgitation with peak flow velocity of 3.2 m/s. The left ventricular pressure decreased to 60% of the right ventricular pressure. The mild tricuspid regurgitation persisted, whereas the right ventricular contraction was maintained 1 month after the operation. The patient had an uneventful recovery and was discharged on post-operative day 36.

Figure 2. Surgical photographs of the Blalock-Taussig shunt ( a – c ). ( a ), Usual atrial situs and D-transposition of the great arteries. ( b, c), The main pulmonary trunk is not hypoplastic, and the lumen was preserved. aAo = ascending aorta; LAA = left atrial appendage; LPA = left pulmonary artery; PT = pulmonary trunk; RAA = right atrial appendage; RPA = right pulmonary artery; RV = right ventricle.
During the outpatient follow-up, the left ventricular morphology gradually changed to a crescent shape (Fig 1d). The left ventricular pressure further decreased, and tricuspid regurgitation worsened from mild to moderate. Cardiac catheterisation at 6 months of age showed mean pulmonary artery pressure of 11 mmHg, which was low enough before the Glenn procedure. The right ventricular end-diastolic pressure was elevated at 8 mmHg, suggesting diastolic dysfunction, and the right ventricular ejection fraction of 51% with preserved contractility.
The bidirectional Glenn procedure and tricuspid valve plasty were performed at 9 months of age. The patient had a good post-operative course and was discharged on post-operative day 36. The patient is currently scheduled to undergo a Fontan procedure.
Discussion
Pulmonary atresia with intact ventricular septum is a relatively common cardiac malformation, but most cases involve concordant ventriculoarterial connections. There have been only three reported cases of pulmonary atresia with intact ventricular septum in the setting of D-transposition of the great arteries. Reference Gupta, Anderson and Kothari1–Reference Hayes, Arya and Kleinman3 These cases were complicated by a hypoplastic mitral valve, mitral stenosis, and hypoplastic left ventricle. They were candidates for single-ventricle palliation. One patient underwent balloon atrial septostomy but was discharged without palliative surgery because the parents did not desire surgical intervention. Another patient died after a systemic-pulmonary shunt, while the final patient underwent heart transplantation due to right ventricular dysfunction. The index case is the first report of a successful Glenn procedure for this rare malformation.
Unlike the previous reports, our patient had severe mitral regurgitation, which required partial closure of the mitral valve. The severe heart failure in the neonatal period was likely caused by increased pulmonary blood flow and myocardial oxygen demand, as well as a compressed right ventricle and severe mitral regurgitation due to high pressure in the left ventricle. Single-ventricle palliation was performed because severe diastolic dysfunction of the left ventricle was suspected due to endocardial fibroelastosis and left ventricular wall thickening. To reduce mitral regurgitation and decompress the left ventricle, partial closure of the mitral valve and Blalock-Taussig shunting were performed simultaneously.
There have been some previous reports on intervention for hypoplastic mitral valve with a hypoplastic left ventricle. Reference Troise, Ranieri and Arciprete4,Reference Napoleone, Formigari, Chiappini, Frascaroli and Gargiulo5 In these cases, mitral exclusion and complete closure were performed. The left ventricular morphology changed to a crescent cavity 2 or 3 weeks after operation. In other case, partial closure of the mitral valve was performed because of the risk of thrombosis, ventricular septal dyskinesia, and late arrhythmia with complete closure of the mitral valve. Reference Menon, Kumar and Mathew6
In our case of severe mitral regurgitation and mild hypoplastic left ventricle, partial closure of the mitral valve was performed, and the left ventricular pressure to 60% of that of the right ventricle. However, the left ventricular morphology gradually changed to a crescent shape, and tricuspid regurgitation worsened to moderate. Hence, tricuspid valve plasty was required during the Glenn procedure.
Thus, partial closure of the mitral valve was useful for decompressing the high-pressure left ventricle and controlling severe mitral regurgitation in this patient with pulmonary atresia with intact ventricular septum in the setting of D-transposition. However, this procedure should be performed carefully to prevent worsening of tricuspid regurgitation due to changes in left ventricular morphology.
Conclusion
The index case is the first report of a successful Glenn procedure for pulmonary atresia with intact ventricular septum in the setting of D-transposition of the great arteries. In cases associated with severe mitral regurgitation and hypoplastic left ventricle, partial closure of the mitral valve may effectively improve heart failure and achieve single-ventricle palliation.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951122000609
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical guidelines for medical and health research involving human subjects and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the institutional review board of the Shizuoka Children’s Hospital.





