Chylothorax occurs in 2.8–3.9% of children following cardiac surgery. Despite its relative infrequency, it is associated with increased mortality, hospital length of stay, cardiac ICU length of stay, time on mechanical ventilation, extracorporeal membrane oxygenation use, and cost.Reference Mery, Moffett and Khan 1 , Reference Buckley, Graham and Gaies 2 In addition, as a result of chylous drainage, these patients are at a higher risk for developing thrombi and poor nutrition.Reference Kocel, Russell and O’Connor 3
Despite several generally accepted treatment modalities, including medium-chain triglyceride feeds/fortified defatted human milk/low-fat diet – medium-chain triglyceride feeds – nothing-by-mouth with enteral nutrition, octreotide, and surgical interventions, there remains a lack of evidence to guide duration and progression of treatments. Consequently, wide practice variation is found in the clinical setting, as well as in the treatment protocols that are available in the literature.Reference Beghetti, La Scala and Belli 4 – Reference Day, Zannino and Golshevsky 11 Our centre is no exception, as discussions with providers and analysis of a cohort of chylothorax patients revealed considerable variation in the treatment of these patients, specifically in the timing and duration of nothing-by-mouth, medium-chain triglyceride feed duration, and a lack of utilisation of additional interventions – octreotide, surgical intervention, etc. Importantly, Yeh et alReference Yeh, Brown and Kellogg 10 demonstrated significant improvement in outcomes in the treatment of postoperative chylothorax following the standardisation of practice through the implementation of a protocol.
Our primary aim was to decrease the duration of postoperative chylothorax in children with CHD, as demonstrated by the duration of chest tube utilisation, through creation and implementation of a chylothorax management protocol.
Materials and methods
Study population and setting
All children undergoing cardiac surgery at Primary Children’s Hospital (February, 2014–October, 2016) who developed a postoperative chylothorax within 30 days of surgery were included. Patients were excluded from analysis if they had congenital chylothorax or had undergone a Fontan operation, as we felt these patients deserved a more aggressive approach. Patients were also excluded if their chylothorax duration was <24 hours, as these effusions probably resolved with little or no influence from the protocol. The study was approved and a waiver of informed consent granted by the University of Utah Institutional Review Board and Primary Children’s Hospital Private Board. The pre-intervention period was from February, 2014 through June, 2015. The cases were selected retrospectively from the cardiothoracic surgery database. After implementation of a chylothorax management protocol, the post-intervention period was from July, 2015 to October, 2016, and cases were identified prospectively.
Definitions
Chylothorax was diagnosed with one or more of the following findings: pleural fluid triglycerides >110 mg/dl, pleural fluid lymphocytes >80%, and/or pleural fluid triglycerides greater than serum triglycerides.
Patient weight used for analysis and in calculations was the patient’s preoperative admission weight in kilograms.
Chest tube output was defined as the cumulative output of all chest tubes for a 24-hour period in relation to the patient’s weight (ml/kg/day).
Low chest tube output (low output) was defined as ⩽20 ml/kg/day during the first 48 hours or <10 ml/kg/day at any time.
High chest tube output (high output) was determined after a 24-hour trial of medium-chain triglyceride feeds and is defined as >20 ml/kg/day. In addition, patients in the low-output arm whose chest tube output remains ⩾10 ml/kg/day after at least 48 hours on medium-chain triglyceride feeds are subsequently redefined as having high output.
Net daily fluid balance was identified by calculating the daily fluid balance – total volume intake minus total volume output – from postoperative day 0 through the date of chylothorax resolution in relation to the patient’s weight (ml/kg/day).
Chylothorax resolution was defined as the day the last chest tube was removed.
Chylothorax recurrence was defined as re-insertion of a chest tube draining diagnostic fluid after chylothorax resolution and within 30 days of resuming full-fat/regular feeds.
Protocol Compliance was affirmative if the instruction was carried out within 24 hours of eligibility. The authors tracked compliance through monthly retrospective chart reviews, rounding on qualifying patients at least every 2–3 days, and contact from providers to discuss rationale for deviations.
Intervention: protocol development and implementation
In December, 2014, literature regarding chylothorax treatment was reviewed at a Cardiac ICU journal club at our institution. The subsequent discussion revealed wide variation in the treatment of chylothorax, including nothing-by-mouth utilisation and duration, duration of medium-chain triglyceride feeds, and lack of surgical intervention. After identifying the need for standardisation, a key driver diagram was developed (Fig 1) using an investigation of 13 patients diagnosed with chylothorax from January to June, 2015. Data analysis confirmed wide variation in the decisions to make a patient nothing-by-mouth (chest tube output ranged from 10–77 ml/kg/day), timing to initiate nothing-by-mouth status (0–5 days), duration of nothing-by-mouth status (1–17 days), and duration of medium-chain triglyceride feeds (2–6 weeks). Out of 13 patients, eight had chest tubes for ⩾10 days, and two of these patients had persistent output >20 ml/kg/day, suggesting that nothing-by-mouth status and medium-chain triglyceride feeds were ineffective, although there were no additional surgical interventions performed on this subset of patients. We concluded that utilising nothing-by-mouth sooner and more rapid progression to surgical intervention would reduce the duration of chest tube utilisation.

Figure 1 Key driver diagram for developing a protocol to reduce chylothorax duration. MCT=medium-chain triglyceride; NPO=nil per os; PDSA=Plan-Do-Study-Act.
The protocol was developed over 6 months (December, 2014 to June, 2015) using literatureReference Beghetti, La Scala and Belli 4 – Reference Yeh, Brown and Kellogg 10 and local expert opinions of a multidisciplinary team that included paediatric cardiothoracic surgeons, cardiologists, cardiac intensive care physicians, nurse practitioners, and registered nurses.
The protocol was implemented on 1 July, 2015 following presentation at a meeting to review new policies related to cardiac patients, with front-line providers in attendance including nurse managers and educators. The protocol was distributed via e-mail, hard copies were placed throughout the cardiac ICU, and electronic copies were made available in a shared drive accessible to cardiac intensive care and cardiology providers. Once a patient was diagnosed with a chylothorax, the protocol was placed in the chart and one of the authors was notified. The authors were available for questions and followed up each patient throughout the hospital course. Iterative interventions occurred using Plan-Do-Study-Act cycles.
Figure 2 displays the current protocol, and Table 1 outlines Plan-Do-Study-Act iterations to arrive at the current protocol. After the diagnosis of chylothorax, all patients are trialed on 24–36 hours of medium-chain triglyceride feeds, defatted human milk fortified with medium-chain triglyceride formula, or a low-fat diet (<10 g/day) before enrolment in the high- or low-output treatment arms. Low chest tube output is managed by providing medium-chain triglyceride feeds for 6 sequential weeks. The high-output state is managed with nothing-by-mouth status for 6 days with re-evaluation every 3 days for re-entry back into the low-output (<10 ml/kg/day) treatment arm. For persistent drainage following nothing-by-mouth for 6 days, a 3-day trial of octreotide is initiated. If chest tube output fails to drop <10 ml/kg/day, the patient will then undergo invasive interventions – thoracic duct ligation, embolisation, or pleurodesis – within 10 days of diagnosis. The invasive intervention is chosen by discussion with the cardiac ICU attending and primary surgeon and depends on patient size, cardiac anatomy, and clinical status. Chest tubes may be removed when output is <3 ml/kg/12 hours.
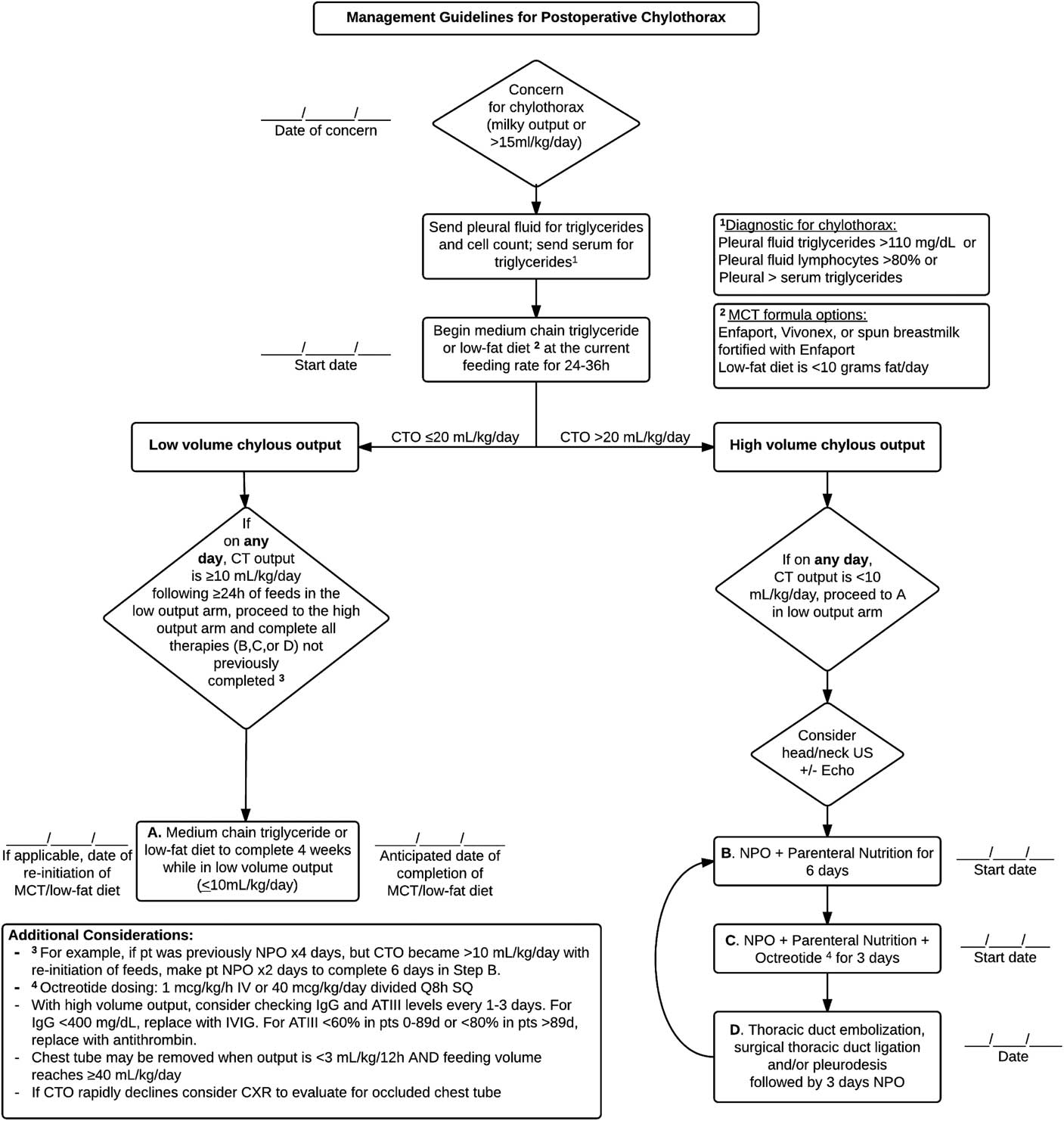
Figure 2 Chylothorax management protocol. ATIII=anti-thrombin III; CT=chest tube; CTO=chest tube output; CXR=chest X-ray; IV=intravenous; IVIG=intravenous immunoglobulin; MCT=medium-chain triglyceride; NPO=nil per os; SQ=subcutaneous; US=ultrasound.
Table 1 Description of Plan-Do-Study-Act cycles.

MCT=medium-chain triglyceride; NPO=nil per os; POD=postoperative day
The protocol also recommends testing levels and replacing anti-thrombin III and immunoglobulin G every 1–3 days in patients with high chest tube output. Values warranting replacement were based upon age-appropriate normal values as determined by our laboratory, suggesting intravenous immunoglobulin replacement for immunoglobulin G levels <400 mg/dl and anti-thrombin administration for anti-thrombin III <60% in patients 0–89 days or <80% in patients >89 days.
Study of the interventions
The first 15 patients in the post-protocol cohort demonstrated no recurrence of chylothorax, and three of these patients had feeds shortened to 2 weeks secondary to feeding intolerance. For these reasons, our first intervention was to reduce the duration of medium-chain triglyceride feeds from 6 to 4 weeks – initiated on 30 May, 2016.
The second intervention – initiated on 15 August, 2016 – involved decreasing the timing for re-evaluation of nothing-by-mouth status from every 3 days to daily. This was based upon the observation that once chest tube output drops <10 ml/kg/day it typically did not increase again regardless of subsequent timing of restarting feeds.
Third, a chylothorax detection algorithm was created to achieve earlier diagnosis – initiated on 15 August, 2016. The algorithm was derived by establishing a chest tube output volume threshold (>15 ml/kg/day; see Results section) for testing pleural fluid rather than relying on milky appearance, which is subjective and may be absent in patients on low-volume or no feeds.
Measures
Our primary intervention was the implementation of a chylothorax management protocol, with outcomes measured by comparing pre- and post-protocol cohorts.
Primary outcomes
The primary outcome of this quality improvement project was chylothorax duration as measured by postoperative duration of chest tube utilisation. Secondary outcomes were postoperative days to chylothorax diagnosis, ICU and hospital lengths of stay, mechanical ventilation days, nothing-by-mouth days, and medium-chain triglyceride feed duration.
Balance measures
Balance measures included chylothorax recurrence as demonstrated by chest tube re-insertion and hospital re-admission related to chylothorax.
Data collection included patient demographics, such as age, sex, race, ethnicity, weight, preoperative cardiac diagnosis, surgical intervention, date of initiation of feeds, date of chylothorax diagnosis, chest tube output, daily fluid balance, nothing-by-mouth days, chest tube days, milrinone use, mechanical ventilation days, head/neck venous ultrasound results, ICU and hospital lengths of stay, re-admission date(s) and diagnosis, additional surgical interventions, and in-hospital mortality.
Statistical analysis
Descriptive statistics were used to provide frequencies and percentages for categorical variables and means, medians, and standard deviations for continuous variables. For discrete variables, we used the χ2 test to determine significance. For continuous variables, we used t-tests assuming unequal variance. A two-sided p-value <0.05 was considered statistically significant. Analyses were performed using Microsoft Excel version 2010.
Results
Clinical characteristics
We analysed a total of 42 patients, divided into pre-protocol (n=20) and post-protocol (n=22) cohorts. Patient characteristics – age, sex, race, ethnicity, weight, and genetics – surgical procedures, pleural triglyceride levels, and daily chest tube output were similar in both cohorts (Table 2).
Table 2 Demographic and clinical characteristics of children diagnosed with chylothorax.

CTO=chest tube output; STAT=The Society of Thoracic Surgeons – European Association for Cardio-Thoracic Surgery Congenital Heart Surgery
Net daily fluid balance (p=0.25) and milrinone use (p=0.29) were also found to be similar across cohorts. On average, chest tubes were removed when 24-hour output was 2.5 ml/kg/day in the pre-protocol cohort and 2.0 ml/kg/day in the post-protocol cohort (p=0.58). Incidence of chylothorax did not change over the study period (pre=4%, post=5%, overall=5%, p=0.66).
Primary and balance measure outcomes
Mean duration of chest tube utilisation decreased significantly from 12 to 7 days (p=0.047). Maximum duration of chest tube utilisation decreased from 44 to 13 days (Fig 3).

Figure 3 Control chart of total days of chest tube utilisation for 42 patients with chylothorax from February, 2014 to October, 2016. A chylothorax management protocol was implemented on 1 July, 2015 – after patient number 20. 1Intervention A was to decrease the duration of medium-chain triglyceride feeds/low-fat diet from 6 weeks to 4 weeks. 2Intervention B was to add a trigger to test for chylothorax when chest tube output was >15 ml/kg/day; also, the need for nothing-by-mouth status was re-evaluated daily instead of every 3 days. UCL=upper control limit.
Postoperative days to diagnosis were unchanged between cohorts (p=0.76). No difference in ICU and hospital lengths of stay or duration of mechanical ventilation was found (Table 3). Mortality was similar across both cohorts. Total nothing-by-mouth duration decreased from 5 to 3 days (p=0.43). As directed by the protocol, medium-chain triglyceride feed duration significantly decreased in the post-protocol cohort (p=0.01).
Table 3 Outcome measures in patients treated for chylothorax.

CLOS=cardiac ICU length of stay; CTU=chest tube utilisation; HLOS=hospital length of stay
No chest tubes re-insertions or re-admissions occurred owing to chylothorax in either cohort, including following any protocol interventions – for example, decreasing medium-chain triglyceride feed duration from 6 to 4 weeks and decreasing interval to re-evaluate chest tube output for ability to resume feeds.
Additional treatment characteristics
Protocol intervention outcomes are further detailed in Table 4. Protocol compliance was 81%. Octreotide and thoracic duct ligation were never used in the pre-protocol cohort. Octreotide – in three patients – and thoracic duct ligation – in two patients – were utilised in the post-protocol cohort. Octreotide was used for 3–6 days and chest tube output remained ⩾20 ml/kg/day on all treatment days, and all three patients underwent additional interventions. Thoracic duct ligation resulted in chylothorax resolution within 24 hours without recurrence in both patients. One patient in the post-protocol cohort underwent balloon dilation of the atrial septum in the setting of elevated right atrial pressures (20 mmHg) with resolution of chylothorax within 2 days.
Table 4 Protocol intervention outcomes in patients treated for chylothorax.

MCT=medium-chain triglyceride feeds/fortified defatted human milk/low-fat diet; NPO=nil per os; POD=postoperative day; US=ultrasound
Head/neck ultrasound was performed in seven pre-protocol patients and eight post-protocol patients. Occlusive thrombus was detected in one pre-protocol and two post-protocol patients. All occlusive thrombi were treated with anti-coagulation. One pre-protocol and four post-protocol patients had femoral vein or artery thrombi related to cardiac catheterisation or central venous lines and were treated with anti-coagulation.
Chylothorax detection algorithm
In analysing chylothorax patients, we found that chest tube output was high even before its appearance turned milky. If the output proved to be significantly higher than non-chylothorax patients, we felt this could help diagnose chylothorax earlier and thus lead to earlier resolution. Therefore, a detection algorithm was created using the chylothorax cohorts and 99 consecutive patients in the post-protocol era who were never diagnosed with chylothorax. Supplementary Table 1 compares mean chest tube output on postoperative days 0–5 for chylothorax and non-chylothorax patients. Analysis revealed that chest tube output >15 ml/kg/day provided 88% sensitivity and 68% specificity for detecting chylothorax. In the 8 weeks since implementation of the algorithm, five patients have been tested for chylothorax. Three patients tested positive for chylothorax after meeting criteria for testing but also had a chest tube output that was milky in appearance. Two patients with output >15 ml/kg/day were tested and found not to have chylothorax.
Discussion
We demonstrated a significant decrease in chylothorax duration as measured by chest tube utilisation in postoperative children who have undergone cardiac surgery through implementation of a single-centre chylothorax management protocol.
The studies by Yeh et alReference Yeh, Brown and Kellogg 10 and Day et alReference Day, Zannino and Golshevsky 11 are the only other published studies that present pre- and post-protocol data in the treatment of chylothorax. In comparison with their results, we saw a shorter post-protocol median chest tube utilisation of 7 versus 14Reference Yeh, Brown and Kellogg 10 and 11.5Reference Day, Zannino and Golshevsky 11 days.
Importantly, our exclusion criteria varied from these studies, which excluded only patients who underwent surgical repairs via lateral thoracotomyReference Yeh, Brown and Kellogg 10 and included all chylothorax patients.Reference Day, Zannino and Golshevsky 11 However, in the study by Yeh et alReference Yeh, Brown and Kellogg 10 , Fontan patients were not included until drainage lasted >1 week, which only occurred in 20 out of 118 patients in the early cohort and 4 out of 45 patients in the late cohort (personal communication).
Two additional studies analysed outcomes while utilising protocols in the management of all postoperative children with CHD who developed chylothorax at their respective institutions.Reference Beghetti, La Scala and Belli 4 , Reference Milonakis, Chatzis and Giannopoulos 7 Beghetti et alReference Beghetti, La Scala and Belli 4 separated patients into several cohorts, and thus we only used the group with CHD with thoracic injury for comparison, which did not include patients with high venous pressures, including Fontan patients. Although these studies lacked pre-protocol measurements for comparison, their post-protocol median and mean chest tube utilisation were longer than our post-protocol results – median duration of 7 versus 18 daysReference Milonakis, Chatzis and Giannopoulos 7 and mean of 7 versus 20 daysReference Beghetti, La Scala and Belli 4 . Thus, to our knowledge, the duration of chest tube utilisation we report following implementation of a protocol at our single institution is the shortest in the reported literature.
Although this study was not designed to demonstrate causality or association of the primary outcome with any given instruction in the protocol, we believe the greatest change in chest tube duration came from the ability to treat patients with high-output, persistent chylothorax more effectively and efficiently. Analysis of demographic and clinical data showed similar cohorts in regard to demographics, surgical procedure, and number of patients with high chest tube output. Despite these similarities, the pre-protocol cohort had 7 (35%) patients with chest tube duration of >10 days, and the post-protocol cohort had 2 (9%). In addition, chylothorax duration was probably decreased owing to shortening duration of therapies once a low-output state is achieved. Specific elements in the protocol that potentially influenced success include reduction in practice variability; initial trial of medium-chain triglyceride feeds; early but minimal use of nothing-by-mouth status and frequent evaluation for low- versus high-output treatment; shortened medium-chain triglyceride feed duration; and early progression to surgical intervention.
Improvements in outcomes have been shown through the standardisation of chylothorax management.Reference Yeh, Brown and Kellogg 10 We similarly saw a reduction in practice variation with 81% compliance to the protocol. All post-protocol patients were appropriately classified as low or high output based upon chest tube output and subsequently reclassified in accordance with the protocol where applicable. The majority of patients were also managed based upon the guidelines of the protocol in regard to duration or medium-chain triglyceride feeds, duration of nothing-by-mouth, and timing of additional intervention. Deviations from our protocol included three patients with medium-chain triglyceride feed duration shortened from 4–6 weeks to 2 weeks owing to formula intolerance. The management of these patients was otherwise directed by the protocol; total chest tube days were 9, 4, and 4, respectively, and none had recurrence of chylothorax. After noting these feeding deviations and the lack of recurrence of chylothorax in these patients, medium-chain triglyceride feed duration was decreased to 4 weeks, resulting in 100% compliance and no increase in adverse events. The other protocol deviation came from a patient who received earlier use of octreotide and surgical intervention after only 5 days of nothing-by-mouth, based on surgeon concern for surgical damage to the thoracic duct. Chylothorax resolved within 24 hours of thoracic duct ligation, the patient had 10 total chest tube days, and chylothorax did not recur.
Medium-chain triglyceride feeds are used for chylothorax treatment because they are absorbed by intestinal cells and transported directly to the liver via the portal vein, thus bypassing the thoracic duct and minimising chylous leakage into the pleural space.Reference Ismail, Kabbani and Najm 12 , Reference McCray and Parrish 13 We postulated that some patients who initially present with high output would transition to a low-output state after medium-chain triglyceride feeds, thereby reducing the number of patients exposed to the more aggressive high-output treatments. Thus, we trial medium-chain triglyceride feeds for 24 hours before committing to a high- or low-output treatment arm. In contrast, other protocols prescribe moving directly to nothing-by-mouth for at least 7 days for any patient who presents with high output.Reference Panthongviriyakul and Bines 8 , Reference Yeh, Brown and Kellogg 10 Our method reduced the number of patients in the high-output arm from 12 to 8, thus eliminating at least 12 nothing-by-mouth days across these four patients. The 12 days represent 29% of total potential and actual nothing-by-mouth days across all patients with high output at the time of diagnosis.
Once a patient entered the high-output treatment arm, they were immediately made nothing-by-mouth. In prior studies, nothing-by-mouth duration is 5–21 days before re-evaluation.Reference Beghetti, La Scala and Belli 4 , Reference Chan, Russell and Williams 6 – Reference Panthongviriyakul and Bines 8 , Reference Yeh, Brown and Kellogg 10 , Reference Nath, Savla and Khemani 14 Despite earlier use, our cumulative nothing-by-mouth days were still much lower than prior studies with a median duration of 3 days compared with 6Reference Yeh, Brown and Kellogg 10 and 7 daysReference Nath, Savla and Khemani 14 . During protocol creation, the decision to shorten nothing-by-mouth duration was guided by an examination of a subset of pre-protocol high-output patients. We noticed that once a low-output state was achieved, eight of nine patients remained in the low-output state after initiation of feeds, regardless of nothing-by-mouth duration, which ranged from 1 to 17 days. The interval to re-evaluation of nothing-by-mouth status was later decreased from 3 days to daily, allowing earlier resumption of enteral nutrition using medium-chain triglyceride feeds. Of the post-protocol patients who were treated with nothing-by-mouth, none had chest tube output ⩾10 ml/kg/day after the resumption of medium-chain triglyceride feeds.
Medium-chain triglyceride feed duration required to prevent recurrence of chylothorax has never been established and reported duration is typically 6 weeks.Reference Chan, Russell and Williams 6 , Reference Milonakis, Chatzis and Giannopoulos 7 , Reference Yeh, Brown and Kellogg 10 However, as directed by our first protocol intervention, medium-chain triglyceride feed duration was decreased from 6 to 4 weeks without an increase in adverse effects. Shortening medium-chain triglyceride feed duration is clinically important as medium-chain triglyceride diets are deficient in nutritional factors present in regular diets and associated with poor weight gain.Reference Kocel, Russell and O’Connor 3 , Reference Densupsoontorn, Jirapinyo and Tirapongporn 15
Another commonly used treatment for persistent chylothorax is octreotide; however, the evidence to support its use for chylothorax management is variable.Reference Chan, Russell and Williams 6 , Reference Caverly, Rausch, da Cruz and Kaufman 16 , Reference White, Seckeler and McCulloch 17 Therefore, we prescribed octreotide only for high-output patients who failed 6 days of nothing-by-mouth therapy. Octreotide use was limited to 3 days compared with prior protocols that advocated for 7–14 days before moving to surgical intervention.Reference Chan, Russell and Williams 6 – Reference Panthongviriyakul and Bines 8 , Reference Yeh, Brown and Kellogg 10 No patient in our study had resolution of chylothorax while using octreotide.
Traditionally, medical management is utilised for at least 3–4 weeks before moving to invasive interventions to treat chylothorax.Reference Beghetti, La Scala and Belli 4 , Reference Chan, Russell and Williams 6 – Reference Panthongviriyakul and Bines 8 However, studies indicate that for persistent high output, earlier surgical intervention is successful and carries a relatively low risk.Reference Nath, Savla and Khemani 14 , Reference Matsuo, Takahashi and Konishi 18 Therefore, similar to Yeh et al,Reference Yeh, Brown and Kellogg 10 our protocol progresses to surgical intervention within 10 days of chylothorax diagnosis. We found this strategy to be successful in the three post-protocol patients that qualified for thoracic duct ligation or percutaneous intervention, with resolution of chylothorax within 24–48 hours. Waiting several more days or weeks could potentially allow chylothorax to resolve without an invasive intervention, but the patients would probably be subject to severe complications related to prolonged high-output drainage, including respiratory insufficiency, infection, poor nutrition and growth, thrombosis, and skin breakdown.Reference Mery, Moffett and Khan 1 – Reference Kocel, Russell and O’Connor 3
Given that no patients in our pre-protocol cohort benefited from octreotide, percutaneous intervention, or thoracic duct ligation, compared with three post-protocol patients, it is plausible that these interventions contributed to our decreased chest tube utilisation. While the number of patients who benefited from these interventions is small, the advantage, as demonstrated by a decrease in maximum duration of chest tube utilisation from 44 to 13 days, is meaningful.
Another goal of protocol implementation was earlier detection of chylothorax. Although there was no change in time to diagnosis, our post-protocol time from surgery to chylothorax diagnosis is lower than other reports with a median of 4 versus 6–8.5 days.Reference Chan, Russell and Williams 6 , Reference Yeh, Brown and Kellogg 10 , Reference Nath, Savla and Khemani 14 We aim to achieve earlier diagnosis of chylothorax by establishing an objective detection algorithm based on volume of chest tube output rather than on the subjective judgement of pleural fluid appearance. To date, only five patients have used the algorithm without improvement in time to post-surgical diagnosis. The detection algorithm will continue to be refined as additional data are gathered, with plans to automate the testing process.
In addition, postoperative management practices exclusive of the chylothorax protocol content may also contribute to our decreased chest tube utilisation. Potential variables include chest tube removal patterns, central venous pressure management, and feeding practices.
Although our protocol recommends more aggressive chest tube removal at 3 ml/kg/12 hours compared with published recommendations of <2 ml/kg/hour,Reference Chan, Russell and Williams 6 , Reference Milonakis, Chatzis and Giannopoulos 7 actual practice at our centre demonstrated that removal occurred on average at 2 ml/kg/day (Table 2). It is unlikely that early removal was a significant factor for our decreased chest tube utilisation.
High central venous pressures are implicated as a factor in causing and/or prolonging chylothorax.Reference Panthongviriyakul and Bines 8 We found no difference in net fluid balance or milrinone use in the pre- and post-protocol cohorts, suggesting that neither intravascular fluid status as measured by fluid balance nor the lusitropic effects of milrinoneReference Chang, Atz and Wernovsky 19 influenced the effectiveness of the protocol.
Chylothorax diagnosis typically occurs as feeding volumes increase, allowing for enough chyle to spill into the pleural space.Reference Tutor 9 Although feeding practices were unchanged between our pre- and post-protocol cohorts, it is possible that our institution advances feedings more quickly, leading to earlier recognition.
Although we achieved our primary aim of decreasing the duration of chylothorax, we did not see a significant decrease in ICU or hospital lengths of stay or duration of mechanical ventilation. Although not statistically significant, we saw a meaningful decrease in hospital length of stay from 30 to 21 days, which equals 198 saved patient days. In comparison with Yeh et al, our post-protocol ICU length of stay is similar – our median of 10 versus theirs of 9 daysReference Yeh, Brown and Kellogg 10 – and our hospital length of stay is 5 days shorter (17 versus 23 daysReference Yeh, Brown and Kellogg 10 ). Data from institutions participating in the Pediatric Cardiac Critical Care Consortium also demonstrate that patients with chylothorax had much higher median lengths of stay, with 17.8 ICU days and 30 hospital days.Reference Buckley, Graham and Gaies 2 In addition, we believe that patients with chylothorax are a medically complex population with many factors causing prolonged length of stay. In post-protocol patients, we noted that chest tubes were removed on average 3 days before transfer out of the ICU and 13 days before hospital discharge, indicating that chylothorax was probably not the primary reason for delayed transfer or discharge. Other potential factors that interplay with chylothorax and influence the length of stay include infections, prolonged respiratory insufficiency, and feeding issues. Future iterations will focus on these other comorbidities and aim to improve length of stay.
Limitations of this study include those inherent to quality improvement projects, such as the sequential nature of the pre- and post-protocol cohorts. Our sample size is small, which is expected with studying a relatively rare event, such as chylothorax, from a single institution. Diuretics are often thought to be an adjunct therapy in the treatment of chylothorax; however, we opted not to direct diuretic use in the protocol because of its interplay between so many other aspects of medical care, including cardiovascular stability, renal function, and feeding status. However, there was generally no change in the overall practice of diuretic use at our institution in the study period, and all pre- and post-protocol patients were managed on diuretics at least through removal of chest tubes. Unfortunately, we could not collect central venous pressure data, as many of the studied patients did not have central lines at the time of chylothorax diagnosis. Although we did not specifically measure central venous pressure or the dosage of diuretics, we did measure daily fluid balance, which is the intended outcome of these variables, and found it to be similar in both cohorts. In addition, we did not delineate the likely aetiology of chylothorax, although we do suggest obtaining head/neck ultrasounds and echocardiograms in patients with high output to investigate for the presence of thrombi and to estimate right heart pressures by doppler. Finally, this protocol excludes patients with congenital chylothorax or who have undergone a Fontan operation, as these patients are prone to develop chylothoraces in isolation from surgical procedures.
Conclusions
Our chylothorax management protocol is associated with shorter duration of chest tube utilisation and prescribes reduced duration of nothing-by-mouth status and medium-chain triglyceride feeds compared with prior protocols without an increase in chylothorax recurrence. Further research is required to determine generalisability to other centres and patient populations.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the University of Utah and Primary Children’s institutional review boards.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951118000392










