Anomalous origin of one pulmonary artery branch is a rare congenital condition that is believed to originate from a single embryological defect in the proximal sixth aortic arch.Reference Kutsche and Van Mierop1–Reference Apostolopoulou, Kelekis, Brountzos, Rammos and Kelekis3 The defects include anomalous origin of one pulmonary artery branch from the aorta and unilateral absence of a pulmonary artery. It can be isolated or combined with other intracardiac malformations.
Unilateral absence of a pulmonary artery refers to the proximal interruption of one pulmonary artery branch, with the distal pulmonary artery being usually intact in the lungs.Reference Bouros, Pare, Panagou, Tsintiris and Siafakas4–Reference Pool, Vogel and Blount6 The incidence is about 1 in 200,000.Reference Apostolopoulou, Kelekis, Brountzos, Rammos and Kelekis3 Isolated unilateral absence of a pulmonary artery is much more uncommon,Reference Kadir, Thekudan, Dheodar, Jones and Carroll7 and isolated right pulmonary artery agenesis is twice as common as left pulmonary artery agenesis.Reference Bouros, Pare, Panagou, Tsintiris and Siafakas4, Reference Kadir, Thekudan, Dheodar, Jones and Carroll7, Reference Ten Harkel, Blom and Ottenkamp8 Given that unilateral absence of a pulmonary artery is so uncommon, this deformity can often be missed on a fetal echocardiogram.
Anomalous origin of one pulmonary artery branch from the aorta is an extremely rare conotruncal malformation, which accounts for only 0.12% of all congenital heart defects.Reference Abu-Sulaiman, Hashmi, McCrindle, Williams and Freedom9–Reference Keane, Maltz, Bernhard, Corwin and Nadas11 It can be classified into proximal or distal forms, depending upon the distance between the anomalous vessel and the aortic valve.
This study aims to improve the diagnostic accuracy, as well as outline the differentiating features of these two rare congenital malformations in the fetal period by comparing the prenatal echocardiographic characteristics.
Materials and methods
A retrospective analysis was conducted for all fetuses diagnosed with CHDs at the Pediatric Cardiovascular Center in China, at Beijing Anzhen Hospital, between June 2012 and December 2018. During this period, a total of 1840 fetuses with CHD were examined in our center, and 12 cases of prenatally diagnosed anomalous origin of one pulmonary artery branch were identified in the Fetal Cardiovascular Program database. Diagnoses of anomalous origin of a pulmonary artery were confirmed by postnatal echocardiography and/or surgery in nine cases and by postmortem autopsy in three cases: two cases of anomalous origin of one pulmonary artery branch from the aorta and one case of unilateral absence of a pulmonary artery. The Beijing Anzhen hospital local ethics committee approved the research protocol, and informed consent was obtained in all cases.
The following prenatal parameters were collected: gestational age at referral, the position of the aortic arch, affected pulmonary artery, referring diagnosis, prenatal sonographic findings at other hospitals, and associated fetal cardiac or extracardiac anomalies. Postnatal medical records such as surgical, ultrasonic, and clinical findings were reviewed as well. Postmortem or postnatal diagnostic confirmation was available for all cases.
All fetal echocardiographic examinations were performed on Philips IE33 or Philips EPIQ 7C Color Doppler ultrasound units (Philips Royal Electronics Corporation, Holland) using 2.0–5.0 MHz convex array probes. Fetal heart examinations were performed in accordance with the 2013 guidelines of the American Institute of Ultrasound in Medicine.12
The long axis view of the left ventricular outflow tract, three vessels and trachea view, and the coronal view of the root of the innominate artery were included in this study. Pulsed and color Doppler interrogation were also conducted to confirm the distal intrapulmonary arteries, arterial duct(s), and left and right pulmonary arteries. All fetal echocardiography examinations were performed and reviewed by two fetal/pediatric cardiologists.
Results
Prenatal findings
All cases were referred to our center for fetal echocardiography at 25–30 weeks of gestation because of suspected cardiac findings. None of the cases had extracardiac findings. All patients had unremarkable family histories and were conceived naturally with uneventful pregnancies prior to diagnosis.
A total of 12 fetuses with anomalous origin of one pulmonary artery branch were diagnosed, including six cases with anomalous origin of one pulmonary artery branch from the aorta and six cases with unilateral absence of a pulmonary artery. Among the six fetuses with unilateral absence of a pulmonary artery, four cases were isolated unilateral absence of the right pulmonary artery, one was isolated unilateral absence of the left pulmonary artery, and the sixth case was unilateral absence of left pulmonary artery combined with tetralogy of Fallot. Among the six fetuses with anomalous origin of one pulmonary artery branch from the aorta, the right pulmonary artery was affected in five cases, including three cases with isolated right pulmonary artery from the aorta and two cases combined with Berry syndrome (a distal aortopulmonary window, an aortic origin of the right pulmonary artery, an interrupted or hypoplastic aortic arch, and patent ductus arteriosus with an intact ventricular septum), and one case, case 12, with anomalous origin of left pulmonary artery from the aorta with tetralogy of Fallot-type absent pulmonary valve syndrome. All cases of anomalous origin of one pulmonary artery branch from the aorta were of the proximal form. Table 1 summarizes the clinical observations.
Table 1. Abnormalities and outcomes of 12 cases with anomalous origin of pulmonary artery branch from the ascending aorta

AOLPA = anomalous origin of left pulmonary artery from the aorta; AORPA = anomalous origin of right pulmonary artery from the aorta; GA = gestational age; MA = maternal age; PA = pulmonary artery; PDO = patent ductus arteriosus occlusion; TGA = complete transposition of great arteries; TOF = tetralogy of Fallot; TOP = termination of pregnancy; UAPA = unilateral absence of pulmonary artery.
Fetal echocardiographic features
The diagram of fetal unilateral absence of the pulmonary artery and proximal form of anomalous origin of one pulmonary artery branch from the aorta is demonstrated in Fig 1. The common sonographic findings include (1) lack of confluence at the bifurcation of the main pulmonary artery on multiple views, with the affected pulmonary branch not attached to the main pulmonary artery and (2) in both conditions, the abnormal origin of the pulmonary artery may either be isolated or associated with other malformations.

Figure 1. (a and b) Diagram of fetal unilateral absence of a pulmonary artery and the proximal form of anomalous origin of the right pulmonary artery. AAO = ascending aorta; BA = brachiocephalic artery; DAO = descending aorta; L-CCA = left common carotid artery; L-DA = left ductus arteriosus; LPA = left pulmonary artery; L-SCA = left subclavian artery; PA = pulmonary artery; R-CCA = right common carotid artery; R-DA = right ductus arteriosus; RPA = right pulmonary artery; R-SCA = right subclavian artery.
The sonographic findings in fetuses with proximal anomalous origin of one pulmonary artery branch from the aorta are characterized by two-dimensional and color Doppler imaging, demonstrating the affected pulmonary artery rising from the right posterior or left posterior wall of the ascending aorta close to the aortic valve on three vessels and trachea view, and the long axis view of the left ventricular outflow tract (Fig 2).

Figure 2. Fetal echocardiogram of case 7 at the time of diagnosis. (a), Long-axis view of the left ventricle demonstrating the right pulmonary artery (RPA) originating from the proximal ascending aorta (AO). (b), Color Doppler demonstrating blood flow in the RPA in continuity with the AO. (c), The three vessels and trachea view (3VT) showing no confluence at the bifurcation of the main pulmonary artery (PA), the left pulmonary artery (LPA) (red arrow) originating from the main PA and the RPA (yellow arrow) originating from the proximal AO. (d), Color Doppler showing the blood flow in the RPA was discontinuous with AAO and the blood flow in the LPA was continuous with the PA. AO = ascending aorta; DA = ductus arteriosus; LA = left atrium; LPA = left pulmonary artery; LV = left ventricle; PA = pulmonary artery; RA = right atrium; RPA = right pulmonary artery; RV = right ventricle; RVOT = right ventricular outflow tract; SP = spine.
The unique sonographic findings in fetuses with unilateral absence of a pulmonary artery include (1) normal blood flow of the affected pulmonary artery branch in the ipsilateral intrapulmonary tissue, as demonstrated by color Doppler; (2) three vessels and trachea view and the coronal view of the base of innominate artery, depicting the affected pulmonary artery branch connecting with the caudal aspect of the innominate artery by the ipsilateral ductus arteriosus; (3) a connection between the distal left pulmonary artery with the ventral side of the aortic arch via the left arterial duct in a fetus with unilateral absence of the pulmonary artery and coexisting tetralogy of Fallot; and (4) absence of the proximal left pulmonary artery is associated with a right-sided aortic arch (and vice versa), whereas absence of the proximal right pulmonary artery is associated with a left-sided aortic arch (Figs 3 and 4). Table 2 summarizes the prenatal sonographic characteristics of these cases of unilateral absence of pulmonary artery and proximal anomalous origin of one pulmonary artery branch from the aorta.
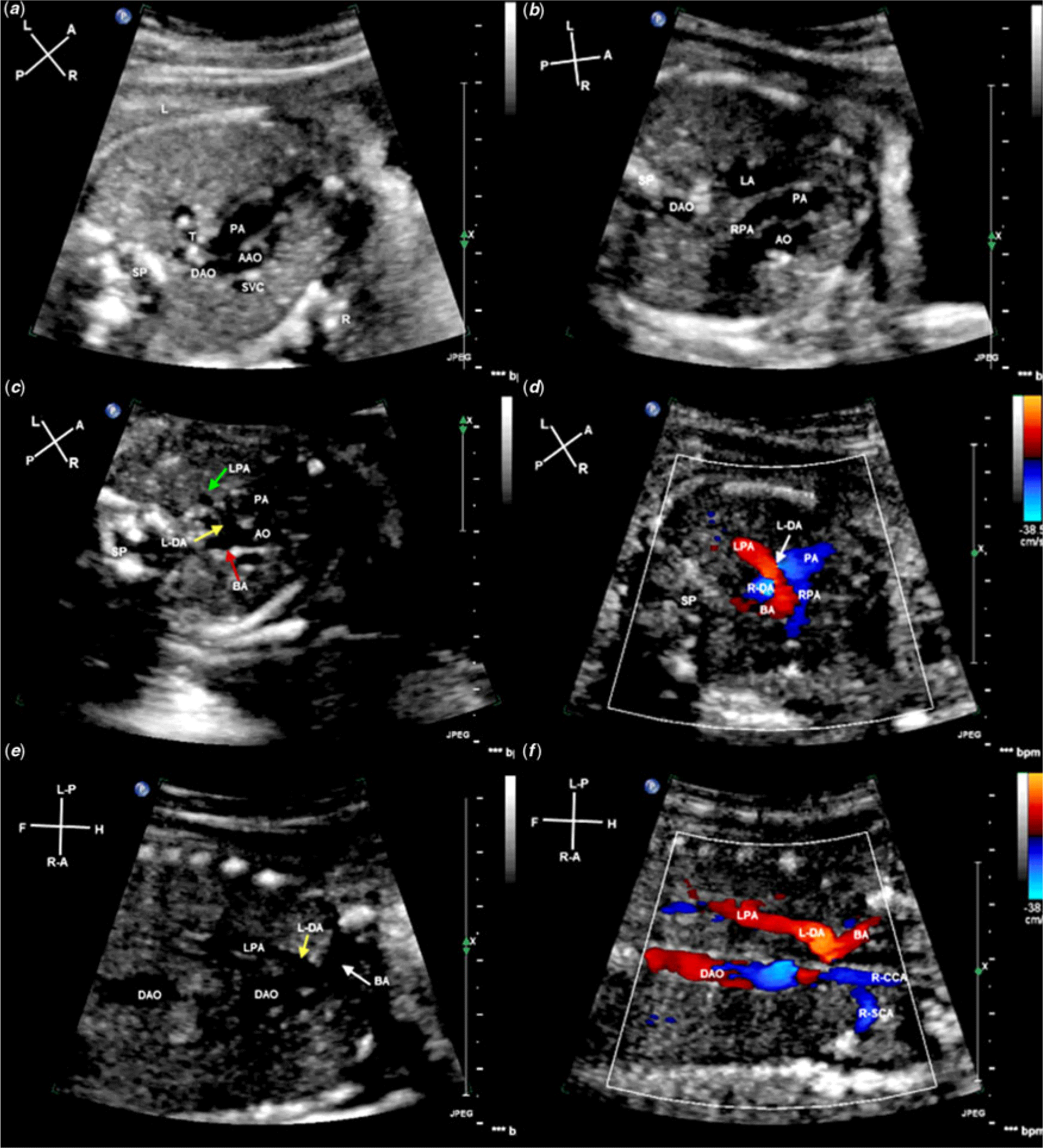
Figure 3. Fetal echocardiogram of case 5 at the time of diagnosis. (a), Three vessels and trachea view (3VT) showing the right aortic arch. (b), 3VT view showing no confluence at the bifurcation of the main pulmonary artery (PA), the right pulmonary artery (RPA) originating from the main PA and the left pulmonary artery (LPA) origin was not clear. (c), 3VT view showing that the left ductus arteriosus (L-DA) (yellow arrow), originating from the base of brachiocephalic artery (BA) (red arrow), is continuous with the LPA (Green arrow). (d), Color Doppler showing isolated blood flow in the LPA and discontinuous flow with the PA. (e), Coronal view of the root of BA showing L-DA (yellow arrow) originating from BA and L-DA continuity with the LPA, the direction of blood flow within the LPA and BA are different. (f), Color Doppler showing that L-DA was continuous with the LPA and the direction of blood flow within the BA, right common carotid artery (R-CCA) and right subclavian artery (R-SCA) toward the right head and neck. AAO = ascending aorta; BA = brachiocephalic artery; DAO = descending aorta; L = left; LA = left atrium; L-CCA = left common carotid artery; L-DA = left ductus arteriosus; LPA = left pulmonary artery; L-SCA = left subclavian artery; LV = left ventricle; PA = pulmonary artery; R = right; RA = right atrium; R-CCA = right common carotid artery; R-DA = right ductus arteriosus; RPA = right pulmonary artery; R-SCA = right subclavian artery; RV = right ventricle; SP = spine; T = trachea.

Figure 4. Fetal echocardiogram of case 1 at the time of diagnosis. (a), Three vessels and trachea view (3VT) showing the left aortic arch. (b), 3VT view showing no confluence at the bifurcation of the main pulmonary artery (PA), the left pulmonary artery (LPA) originating from the main PA and the right pulmonary artery (RPA) origin was not clear. (c), Coronal view of the root of BA showing the right ductus arteriosus (R-DA) originating from the AAO and R-DA in continuity with the RPA. (d), Color Doppler showing that the R-DA (white arrow) was in continuity with the RPA. (e), Color Doppler showing blood flow in the RPA and R-DA (white arrow) was toward the right lung, and the direction of BA was toward the right head and neck on the coronal view of the root of BA. (f), Spectral Doppler showing the high resistance blood flow spectrum of the vessel in the lung, which further confirmed the vessel was the RPA. AAO = ascending aorta; AO = aorta; BA = brachiocephalic artery; DAO = descending aorta; L = left; L-DA = left ductus arteriosus; LPA = left pulmonary artery; PA = pulmonary artery; R = right; R-CCA = right common carotid artery; R-DA = right ductus arteriosus; RPA = right pulmonary artery; SP = spine; SVC = superior vena cava; T = trachea.
Table 2. Prenatal sonographical characteristics of UAPA and proximal AOPA

3VT = three vessels and trachea view; AAO = ascending aorta; AOPA = anomalous origin of one pulmonary artery branch from the aorta; DA = ductus arteriosus; LV = left ventricular; PA = pulmonary artery; UAPA = unilateral absence of pulmonary artery; + = positive; − = negative.
Postnatal follow-up
Cases 2, 8, and 12 were induced at 28, 26, and 25 weeks of gestation, respectively. The autopsy examination of cases 2, 8, and 12 confirmed the prenatal diagnosis. The other nine fetuses were spontaneously delivered at full term without any complications. In case 10 with Berry syndrome, the diagnosis was confirmed with postnatal echocardiography. The patient died at 63 days postpartum without surgical intervention from recurrent pneumonia and heart failure.
The ipsilateral connecting arterial duct closed at 3 days after birth in case 1, by 1 month in cases 3 and 5, and by age 2 months in cases 4 and 6. None of the unilateral absence of pulmonary artery patients underwent early pulmonary artery reconstruction in this study. All natal unilateral absence of pulmonary artery cases were confirmed by the postnatal echocardiography examinations, cardiac-enhanced CT examinations, or surgery for cardiac anomalies.
Case 1 underwent contralateral patent ductus arteriosus occlusion 6 months after birth due to pulmonary arterial hypertension. Case 6 was treated with radical surgery for tetralogy of Fallot 12 months after birth. Right pulmonary artery reconstruction was performed in cases 7, 9, and 11 approximately 2–3 months after birth. These patients were discharged from the hospital without complications 2–15 days after surgery. They remained well with no evidence of pulmonary arterial hypertension during the follow-up period from 3 months to 3 years and had no indications for further interventions. All surviving cases of isolated unilateral absence of pulmonary artery cases developed normally with no evidence of pulmonary arterial hypertension or other symptoms at 2-year follow-up. Chromosomal analyses were normal (46XX or 46XY) in all postnatal cases, but chromosome 22q11.3 deletion was not studied.
Discussion
Unilateral absence of a pulmonary artery is a rare cardiac malformation.Reference Apostolopoulou, Kelekis, Brountzos, Rammos and Kelekis3 It refers to the proximal interruption of the pulmonary artery, with no distal abnormality, as the intrapulmonary vessel is relatively normal in distribution in the lungs.Reference Pool, Vogel and Blount6 The distal intrapulmonary vessel is typically supplied by an ipsilateral patent ductus arteriosus.Reference Bouros, Pare, Panagou, Tsintiris and Siafakas4–Reference Pool, Vogel and Blount6 If the connecting arterial duct closes after birth, the ipsilateral distal intrapulmonary pulmonary artery will lose its blood supply and will not be visible with ultrasonic imaging. The affected lung will become hypovascular and recruit collaterals from bronchial and other systemic branches.Reference Welch, Hanley, Johnston, Cailes and Shah13 In all reported unilateral absence of pulmonary artery cases with satisfactory angiographical, surgical, or autopsy documentations, it has been pointed out that an arterial duct or ligamentum is found to be located between the root of innominate artery and the ipsilateral distal intrapulmonary vessels.Reference Welch, Hanley, Johnston, Cailes and Shah13 Understanding the anatomical structure and connection of the arterial duct is critical to the diagnosis of unilateral absence of pulmonary artery in the fetal period.
In keeping with the accepted embryological principles, an absent proximal pulmonary artery is caused by the involution of the proximal sixth aortic arch (destined to become the proximal pulmonary artery) and persistence of the connection of the intrapulmonary pulmonary artery to the distal sixth aortic arch(destined to become the arterial duct).Reference Apostolopoulou, Kelekis, Brountzos, Rammos and Kelekis3 The ductus arteriosus connects to the primitive dorsal aorta, becoming the underside of the aortic arch ipsilateral to the arch, or the base of the innominate artery contralateral to the arch.Reference Apostolopoulou, Kelekis, Brountzos, Rammos and Kelekis3 Therefore, the absent proximal pulmonary artery is on the side opposite to the aortic arch,Reference Pfefferkorn, Löser and Pech2, Reference Bockeria, Makhachev, Khiriev and Abramyan14 which was concordant with findings from this study. Unilateral absence of pulmonary artery may be isolated or seen in association with other congenital heart defects, including tetralogy of Fallot, truncus arteriosus, and so on.Reference Kucera, Fiser, Tůma and Hucin15, Reference Szwast, Tian and McCann16
The anomalous pulmonary artery branch from the aorta originates from the wall of the ascending aorta, 5–30 mm above the ventriculo-arterial junction, and may be isolated or associated with other congenital cardiovascular anomalies, including tetralogy of Fallot, Berry syndrome, and so on.Reference Kirklin, Barratt-Boys and Kouchoukos17–Reference Cheng, Xiao, Zhong and Wen21 Depending on the distance between the anomalous origin and the aortic valve, it is classified into the proximal form (close to the aortic valve) and the distal form (close to the right innominate artery). Additionally, it is 5–6 times more likely that the affected artery will be the right pulmonary artery, than the left pulmonary artery.Reference Abu-Sulaiman, Hashmi, McCrindle, Williams and Freedom9–Reference Keane, Maltz, Bernhard, Corwin and Nadas11 In this study, the affected proximal pulmonary artery arose from the right or left posterolateral, or posterior wall of the ascending aorta, in five cases with an affected right pulmonary artery and the one case with an affected left pulmonary artery.
In this study, all cases of anomalous origin of one pulmonary artery branch from the aorta were of the proximal form. The authors believe that the distal form of anomalous origin of one pulmonary artery branch from the aorta and unilateral absence of pulmonary artery may be the same congenital cardiovascular anomaly. This hypothesis is first supported by the fact that the anomalous vessel in unilateral absence of pulmonary artery originated at the root of innominate artery, which is a shared feature of the distal form of anomalous origin of one pulmonary artery branch from the aorta. Second, current embryological theories suggest that the proximal form and distal form have different embryogenesis. Although the embryogenesis of the distal form is unclear, based upon accepted embryological principles, it may be attributed to the development of the right fifth arch and the involution of the proximal pulmonary artery and distal sixth arch.Reference Kutsche and Van Mierop1 In short, the position of the affected vessel, which is located between the distal pulmonary artery and the base of the innominate artery contralateral to the arch, is a ductus arteriosus and cannot be a pulmonary artery. Third, in several cases of the distal form, the anomalous right pulmonary artery had a narrowed site that was usually distant from its origin before surgery.Reference Kutsche and Van Mierop1, Reference Yoo, Moes, Burrows, Molossi and Freedom22–Reference Kajihara, Imoto and Sakamoto25 The behavior of the narrowed proximal pulmonary artery is similar to that of the ductus arteriosus. This may explain the narrowing that is present in some cases with the distal form after birth, or re-narrowing shortly after direct anastomosis surgery. Yoo et alReference Yoo, Moes, Burrows, Molossi and Freedom22 reported that the distal form is often complicated by pulmonary artery stenosis. Nakamura et alReference Nakamura, Yasui, Kado, Yonenaga, Shiokawa and Tokunaga23 reported that one patient with the distal form needed reoperation 1 month after the initial operation because of progressive right pulmonary artery stenosis at the anastomotic site, and graft interposition was performed after exclusion of the stenotic region. Subsequent histopathologic examination of the stenotic region was very similar to findings for an arterial duct.
We compared the prenatal echocardiographic characteristics of the proximal form and those of unilateral absence of a pulmonary artery. The main similarity is the absence of confluence at the bifurcation of the main pulmonary artery. The main difference is that the origin of the anomalous vessel is either from the subclavian artery or directly from the aorta. By analyzing the prenatal echocardiographic characteristics, the authors found that the three vessels and trachea view and the long axis of the left ventricular outflow tract, combined with two-dimensional and color Doppler, offered the best views for displaying the affected proximal left or right pulmonary artery originating from the posterolateral or posterior wall of the ascending aorta near the aortic valve. During the fetal period, it was difficult to display the abnormal origin of the pulmonary artery branch and its relationship with other ipsilateral vessels on conventional views in unilateral absence of pulmonary artery cases. In the three vessels and trachea view, by moving the probe up and down to make a transverse scan, two-dimensional and color Doppler showed a large vessel (innominate artery) originating from the aortic arch. In this view, it was difficult to determine whether this vessel was an aortic or pulmonary artery branch. However, on the coronal view of the large vessel (innominate artery), two-dimensional and color Doppler clearly identified the large vessel originating from the aortic arch was in fact the innominate artery. Also noticeable was the connection between the pulmonary artery branch and the innominate artery by the ipsilateral vertical arterial duct originating from the root of the innominate artery, as well as the left pulmonary artery connected to the ventral side of the aortic arch by the left arterial duct in the fetus with unilateral absence of the pulmonary artery and tetralogy of Fallot.Reference Pfefferkorn, Löser and Pech2, Reference Bockeria, Makhachev, Khiriev and Abramyan14, Reference Han, Yu, Hao, Gao, Weng and He26
Isolated unilateral absence of a pulmonary artery is often asymptomatic during early childhood, and a diagnosis may be delayed until adulthood.Reference Bouros, Pare, Panagou, Tsintiris and Siafakas4 Upon closure of the arterial duct, the blood flow to the affected lung will be diminished, and the intrapulmonary portion of the artery will become hypoplastic. When symptoms do develop, they include shortness of breath, chest pain, recurrent pneumonia, and pulmonary arterial hypertension of the contralateral lung.Reference Apostolopoulou, Kelekis, Brountzos, Rammos and Kelekis3, Reference Bouros, Pare, Panagou, Tsintiris and Siafakas4, Reference Kadir, Thekudan, Dheodar, Jones and Carroll7, Reference Kruzliak, Syamasundar, Novak, Pechanova and Kovacova27 While there is no consensus regarding the treatment of isolated unilateral absence of a pulmonary artery, early detection and surgical repair can provide restoration of a physiological pulmonary circulation, regression of pulmonary arterial hypertension, and potentially normal development of the distal pulmonary vasculature. Unfortunately, no patients in this study with unilateral absence of a pulmonary artery underwent pulmonary artery reconstruction after birth due to financial constraints and, thus far, no successful cases of reconstruction have been reported in China.
In cases with anomalous origin of one pulmonary artery branch from the aorta, clinical symptoms present early in infancy with respiratory distress or congestive heart failure due to the large left-to-right shunt at the systemic-pulmonary level or pulmonary arterial hypertension.Reference Fontana, Spach, Effmann and Sabiston28 Early surgical intervention, preferentially before 12 months of age, is critical to prevent the development of irreversible pulmonary vascular disease.Reference Benatar, Kinsley, Milner, Dansky, Hummel and Levin29 In this study, cases 7, 9, and 11 underwent surgery 2 to 3 months after birth and recovered well during the 3-year follow-up, with no subsequent development of pulmonary arterial hypertension.
As early surgical intervention is critical to the outcome, and accurate prenatal diagnosis of these anomalies should optimally be made during routine obstetric ultrasound examinations.Reference Yoo, Moes, Burrows, Molossi and Freedom22, Reference Li, Mu, Li and Weng30 For example, in the case of proximal anomalous origin of one pulmonary artery branch from the aorta, the normal aorta could be mistaken for the “main pulmonary artery with branches” and could be misdiagnosed as transposition of the great arteries.Reference García-Delgado, Jiménez and Falcón31
In conclusion, for comprehensive understanding of abnormal pulmonary artery branch connections and postnatal pathophysiological changes in fetuses diagnosed with anomalous origin of one pulmonary artery branch from the aorta and unilateral absence of a pulmonary artery, the three vessels and trachea view, the long axis of the left ventricular outflow tract, and the coronal view of the root of innominate artery are important views for displaying the anomalous origin of one pulmonary artery in the fetal period.
Acknowledgments
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The Beijing Anzhen hospital local ethics committee approved the research protocol, and informed consent was obtained in all cases.








