Persistence of the embryological fifth aortic arch in mammals has been the subject of debate for over 100 years, and controversy still exists over its true derivation.Reference Bamforth, Chaudhry and Bennett 1 The first clinical case report ascribed to persistence of the fifth aortic arch in a human being was published by Van Praagh in 1969, as a case of “congenital double-lumen aortic arch”.Reference Van Praagh and Van Praagh 2 A small number of case reports followed,Reference Izukawa, Scott, Durrani and Moes 3 – Reference Gerlis, Dickinson, Wilson and Gibbs 10 until the retrospective analysis of over 2000 cardiopathological specimens at the Royal Brompton Hospital, London, and Killingbeck Hospital, Leeds, proposed persistence of the fifth aortic arch in six specimens, an incidence of one in every 330 autopsy cases.Reference Gerlis, Ho, Anderson and Da Costa 11 Three of the six cases had been diagnosed as persistent fifth aortic arches in vivo. Freedom et alReference Freedom, Yoo, Mikailian and Williams 12 subsequently collated existing case reports and defined four different subtypes based on anatomical and physiological characteristics (Table 1). In the current era, the label itself carries many embryological controversiesReference Gupta, Gulati and Anderson 13 ; however, for the clinician, this group of conditions may explain various unusual anatomical features that may be important to recognise when caring for children with CHD.
Table 1 Types of persistent fifth aortic arch according to Freedom et al.Reference Freedom, Yoo, Mikailian and Williams 12

* The embryological origins of this type of persistent fifth aortic arch are controversialReference Gupta, Gulati and Anderson 13
Classification
The most comprehensive definition of a persistent fifth aortic arch is that offered by Gupta et alReference Gupta, Gulati and Anderson 13 : an extrapericardial vessel arising from the ascending aorta proximal to the origin of the brachiocephalic arteries, terminating either in the dorsal aorta or in the pulmonary arteries via the persistently patent arterial duct. A comparison of these features with other similar structures is offered in Table 2. In this article, for practical purposes, we have also used descriptive classifications relating the proximal and distal connections of the vessel and the direction of blood flow, corresponding to the original subtypes defined by Freedom (Table 1).Reference Freedom, Yoo, Mikailian and Williams 12
Table 2 Comparison of persistent fifth aortic arch (PFAA) and its variants with other vascular abnormalities.

* This embryological origins of this type of PFAA are controversialReference Gupta, Gulati and Anderson 13
Systemic-to-systemic persistent fifth aortic arch
The embryological origins of this form remain particularly controversial.Reference Gupta, Gulati and Anderson 13 It was originally described as “type 1” in the Freedom classification,Reference Freedom, Yoo, Mikailian and Williams 12 with a distal connection to the descending aorta effectively creating a second aortic channel alongside the fourth – that is, the definitive aortic – arch. This was the “double-lumen” or “double-barrelled” aortic arch originally described by Van Praagh,Reference Van Praagh and Van Praagh 2 although it may in fact be related to the formation of dorsal collateral arteries in the fetus rather than to a true persistence of the fifth aortic arch.Reference Bamforth, Chaudhry and Bennett 1 Nevertheless, in clinical practice, it is important to recognise the possibility of a “double-barrelled” aortic arch as being distinct from the classical double aortic arch – that is, bilateral persistence of the fourth aortic arch – in that both arches are usually located on the same side of the trachea. Unlike a “vascular ring” there is therefore no anatomical substrate for mediastinal compression. An isolated double-barrelled aortic arch can be of no haemodynamic significance, and several case reports have been diagnosed incidentally.Reference Geva, Ray, Santini, Van Praagh and Van Praagh 14 , Reference Kirsch and Julsrud 15
In cases of interruptionReference Atsumi, Moriki, Sakakibara, Mitsui, Horigome and Kamma 16 – Reference Uysal, Bostan and Cil 20 or severe coarctationReference Santoro, Caianiello, Palladino, Iacono, Russo and Calabro 21 , Reference Yoshii, Matsukawa, Hosaka, Ueno and Tsuji 22 of the fourth – that is, the definitive – arch, the presence of an additional vascular channel to the distal aorta is critical for survival. In most cases this is provided by persistence of the arterial duct, physiological closure of which leads to a precipitous decline in blood flow to the descending aorta, reduced lower-limb pulses, and death in the early neonatal period if untreated. In the presence of a second aortic channel, however, there may be a normal haemodynamic load on the left ventricle and no difference in the oxygen saturations between the upper and lower limbs. These channels may also be less likely to occlude completely than an arterial duct postnatally, and thus may explain several case reports of otherwise critical aortic arch obstruction that appear to have remained asymptomatic well beyond the neonatal periodReference Zhao, Su, Liu, Cao and Ding 18 , Reference Uysal, Bostan and Cil 20 and even into adulthood.Reference Isomatsu, Takanashi, Terada and Kasama 17 Similar structures have also been described in the neonatal period, identified alongside severe malformations such as transposition of the great vesselsReference Zhao, Su, Liu, Cao and Ding 18 and the common arterial trunk.Reference Lim, Kim, Kim, Lee, Kim and Kim 23 , Reference Parmar, Pillai, Kulkarni and Sivaraman 24
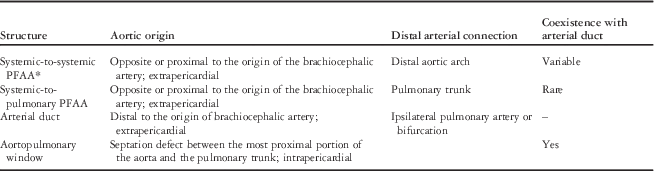
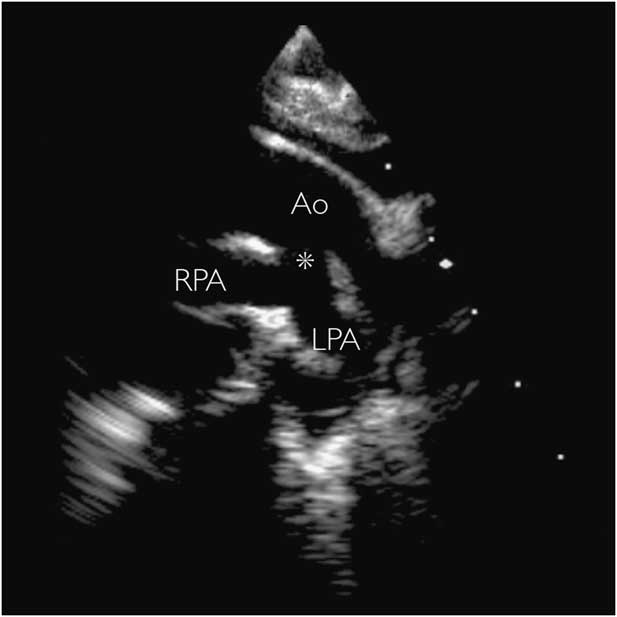
Identifying such channels may be straightforward if the definitive aortic arch is also readily identifiable; however, if there is concomitant interruption of the (fourth) aortic arch it is possible that this connection may appear as the sole aortic arch, making in vivo diagnosis extremely challenging. The appearance would be of an aortic arch in an unusually inferior position, approaching the same level as the main pulmonary trunk and arterial duct, with all head and neck vessels originating from a single connection to the ascending aorta, as shown in Figure 1. Clearly, a similar configuration could also be attributed to a common origin of the brachiocephalic arteries; Gupta et alReference Gupta, Gulati and Anderson 13 describe the potential for unequal growth of the medial and lateral surfaces of the aorta that could account for this from an embryological perspective. The same group posits that only a vascular origin “unequivocally proximal to the origin of the brachiocephalic arteries” can fully justify interpretation as a fifth arch artery. Non-invasive cross-sectional and three-dimensional reconstructions may be able to show this relationship more clearly than echocardiography alone (Figs 2 and 3)Reference Gupta, Gulati and Anderson 13 , Reference Zhong, Jaffe, Zhu, Sun, Li and Gao 25 ; however, even these methods may not always be conclusive.

Figure 1 Two-dimensional transthoracic echocardiography in the suprasternal view showing an interrupted aortic arch with coarctation of a systemic-to-systemic “persistent fifth aortic arch” (asterisk) in a 9-year-old girl presenting with exercise intolerance and reduced volume femoral pulses. AAo=ascending aorta; BCA=brachiocephalic artery; DAo=descending aorta; LCCA=left common carotid artery; LSCA=left subclavian artery.

Figure 2 CT three-dimensional reconstruction of an interrupted aortic arch with distal coarctation of a systemic-to-systemic “persistent fifth aortic arch” (asterisk) in a 9-day-old neonate. The child presented in shock with no response to intravenous prostaglandins, eventually undergoing successful surgical repair. AAo=ascending aorta; DAo=descending aorta.
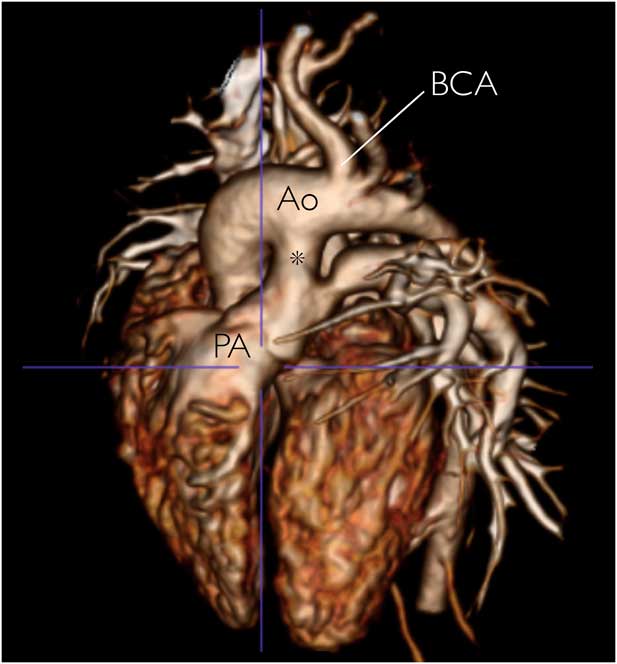
Figure 3 CT reconstruction showing an isolated systemic-to-pulmonary persistent fifth aortic arch (asterisk) in a neonate diagnosed antenatally. Successful surgical repair was performed at 4 months of age.Reference Jowett, Rubens, Ho, Uemura and Gardiner 30 Ao=aorta; BCA=brachiocephalic artery; PA=pulmonary artery. (Reprinted from JACC: Cardiovascular Imaging, 5 (12), Victoria Jowett, Michael Rubens, Siew Yen Ho, Hideki Uemura, Helena M.Gardiner, Prenatal Visualization of Persistent 5th Aortic Arch Artery, 1288-1289, Copyright 2012, with permission from Elsevier.)
Despite the difficulties in diagnosis, clinically the distinction remains important, as both earlyReference Culham and Reed 8 , Reference Uysal, Bostan and Cil 20 – Reference Yoshii, Matsukawa, Hosaka, Ueno and Tsuji 22 , Reference Tomita, Fuse and Chiba 26 , Reference Suda, Matsumura and Matsumoto 27 and lateReference Isomatsu, Takanashi, Terada and Kasama 17 coarctation of these structures has been described. As such, long-term follow-up may be indicated in patients with similar arch appearances even in the absence of obstruction at presentation. Further, in the neonatal period the response to intravenous prostaglandins may be unpredictable, probably depending on the extent of infiltration of the fifth arch with prostaglandin-sensitive ductal tissue. Several case reports have shown restoration of flow in putative “fifth aortic arches” after commencing prostaglandins,Reference Atsumi, Moriki, Sakakibara, Mitsui, Horigome and Kamma 16 , Reference Carroll, Ferris, Chen and Liberman 19 whereas others have observed failure to improve arterial patency, thus requiring urgent surgical intervention.Reference Culham and Reed 8 , Reference Santoro, Caianiello, Palladino, Iacono, Russo and Calabro 21 , Reference Lim, Kim, Kim, Lee, Kim and Kim 23 , Reference Suda, Matsumura and Matsumoto 27
Systemic-to-pulmonary persistent fifth aortic arch
In this type of persistent fifth aortic arch, described as “type 2” by FreedomReference Freedom, Yoo, Mikailian and Williams 12 , the arterial connection is between the ascending aorta and the pulmonary arteries. It is for this group that the embryological evidence for genuine persistence of the fifth arch artery is most convincing.Reference Gupta, Gulati and Anderson 13 This connection could potentially present in several different ways. First, the fifth aortic arch can act as a stand-alone pathological entity causing excessive pulmonary blood flow with pulmonary hypertension. Reports of isolated symptomatic systemic-to-pulmonary persistent fifth aortic arch have only been described relatively recently,Reference Chiu, Wu, Chen and Lin 28 – Reference Jowett, Rubens, Ho, Uemura and Gardiner 30 possibly being under-recognised in the past because of the almost identical presentation to that of isolated patent arterial duct. The arterial duct is, however, always distal to the origin of the brachiocephalic artery, and major aortopulmonary collateral vessels in this region would be highly unusual.Reference Boshoff and Gewillig 31
A systemic-to-pulmonary persistent fifth aortic arch also has the potential to act as a vital aortopulmonary connection when associated with critical pulmonary obstructive lesions such as pulmonary atresia,Reference Yoo, Moes, Burrows, Molossi and Freedom 32 – Reference Lee, Tsao, Wang, Lee and Chiu 34 tetralogy of Fallot,Reference Donti, Soavi, Sabbatani and Picchio 35 and an isolated left pulmonary artery.Reference Lee, Tsao, Wang, Lee and Chiu 34 , Reference Koch, Ludwig, Zink and Singer 36 Systemic-to-pulmonary persistent fifth aortic arch in this context has been associated with abnormalities of chromosome 22q11.Reference Lee, Tsao, Wang, Lee and Chiu 34 , Reference Lee, Chen and Chen 37 As with the systemic-to-systemic form, case reports exist where patency has been maintained with intravenous prostaglandin.Reference Zartner, Schneider and Bein 38 This may well be a function of varying degrees of infiltration of prostaglandin-sensitive ductal tissue within the fifth arch lumen, which has been demonstrated in histopathological studies.Reference Atsumi, Moriki, Sakakibara, Mitsui, Horigome and Kamma 16
Pulmonary-to-systemic persistent fifth aortic arch
In this type of persistent fifth aortic arch, blood flow is pulmonary-to-systemic. Freedom classified this type as type 3, as, although the anatomical connection is the same as type 2, the flow of blood is in the opposite direction. Theoretically, this type could co-exist with any left-sided obstructive lesion; however, only four cases have been described, all of which consisted of the usually non-viable combination of aortic atresia and interrupted aortic arch.Reference Freedom, Yoo, Mikailian and Williams 12 Survival was dependent on a pulmonary-to-systemic channel supplying the segment of ascending aorta that would otherwise be isolated from the circulation, with the descending aorta supplied by a persistent arterial duct. Unfortunately, the small number of cases makes it difficult to accept these malformations as true persistence of the fifth aortic arch with any degree of confidence.
Bilateral persistent fifth aortic arch
Described as “type 4” by Freedom, bilateral persistent fifth aortic arches have only been described on one occasion in a patient with double-outlet right ventricle, subaortic ventricular septal defect, and right aortic arch. In this case, an additional right-sided double-lumen aortic arch coexisted with a left-sided vessel supplying an isolated left pulmonary artery; both were attributed to persistent fifth aortic arches.Reference Wang, Wu and Yang 39 With only a single case report in the literature, this description has also been contested.Reference Gupta, Gulati and Anderson 13
Diagnostic imaging
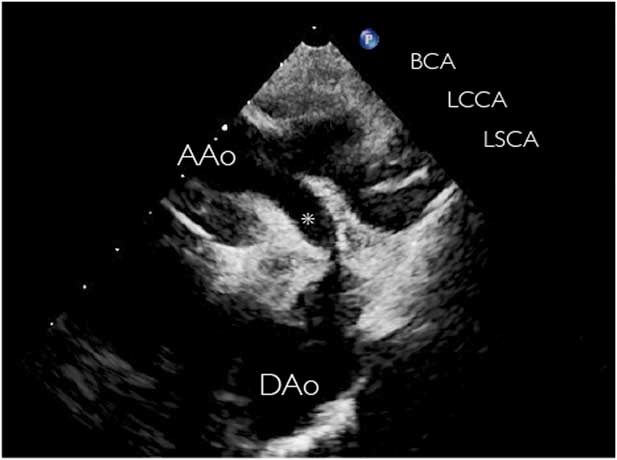
Diagnosis of “persistent fifth aortic arch” using echocardiography alone has been reported.Reference Donti, Soavi, Sabbatani and Picchio 35 , Reference Bernheimer, Friedberg, Chan and Silverman 40 These vessels, although tortuous, would be parallel with the ipsilateral fourth aortic arch, at least in their more proximal portion, and therefore would be most easily seen from the classic “arch” views – that is, from the high-parasternal or suprasternal views. This view may also demonstrate a distal connection to the descending aorta (Fig 1), whereas clockwise rotation to the short-axis view would demonstrate a distal connection to the pulmonary arteries (Fig 4). Three-dimensional reconstruction of cross-sectional images from MRIReference Kirsch and Julsrud 15 , Reference Zhong, Jaffe, Zhu, Sun, Li and Gao 25 or CTReference Tehrai, Saidi and Goudarzi 41 may further elucidate these vascular connections (Figs 2 and 3).
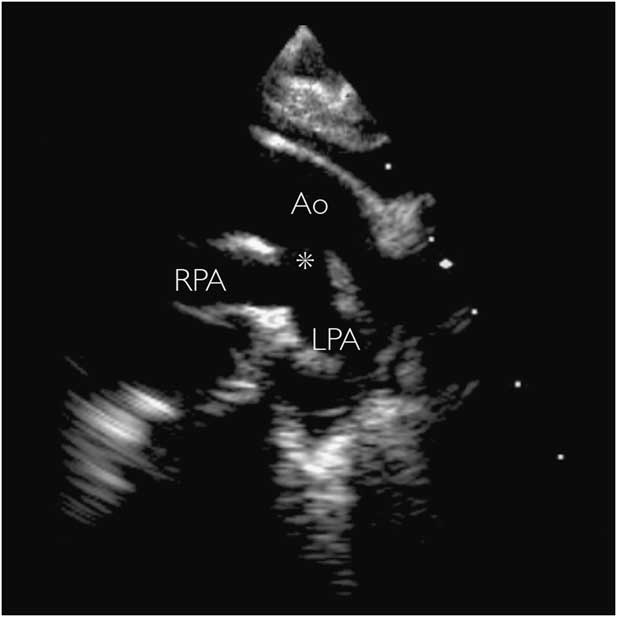
Figure 4 Two-dimensional transthoracic echocardiogram in the suprasternal view in a neonate with pulmonary atresia. Pulmonary blood supply was via a large systemic-to-pulmonary persistent fifth aortic arch (asterisk). Vessel patency was maintained without prostaglandins, and surgical repair was performed at 10 months of age. Ao=aorta; LPA=left pulmonary artery; RPA=right pulmonary artery.
An ascending aortogram performed during diagnostic cardiac catheterisation can provide excellent anatomical information in cases of suspected persistent fifth aortic arch (Fig 5). The speed and accuracy of diagnosis of this method is offset by the need for general anaesthesia, invasive vascular access, and the use of ionising radiation and intravenous contrast. It may not be a suitable option in unstable patients. As well as obtaining diagnostic information, interventional procedures may also be able to be carried out in some cases.Reference Carroll, Ferris, Chen and Liberman 19
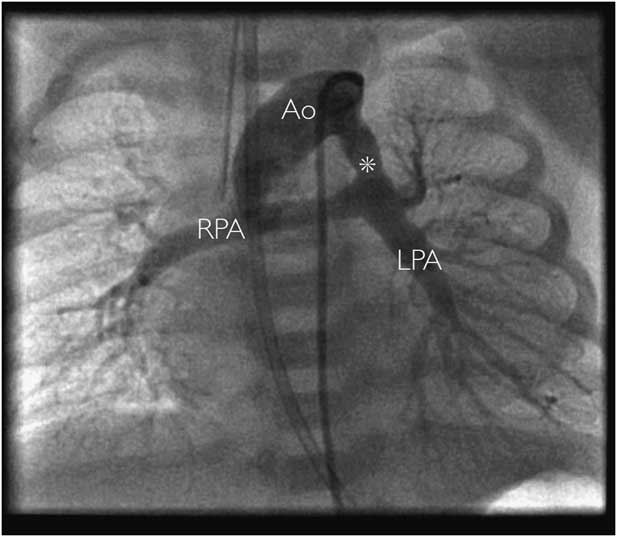
Figure 5 Contrast angiography in a patient with pulmonary atresia demonstrating systemic-to-pulmonary persistent fifth aortic arch (asterisk) connecting the aorta (Ao) to the bifurcation of the pulmonary arteries. In this case, the oxygen saturations dropped precipitously without intravenous prostaglandins, and a surgical aortopulmonary shunt was inserted in the neonatal period. LPA=left pulmonary artery; RPA=right pulmonary artery.
Table 3 describes the clinical presentation, imaging findings, and outcomes of five patients with anatomical findings in which “persistent fifth aortic arch” would be a valid differential diagnosis, according to the definition offered by Gupta et al.Reference Gupta, Gulati and Anderson 13
Table 3 Cases diagnosed in vivo 2005–2014.

AD=arterial duct; AN=antenatal diagnosis; BCA=brachiocephalic artery; CoA=coarctation of the aorta; IAA=interrupted aortic arch; LCCA=left common carotid artery; LPA=left pulmonary artery; L/RmBTS=left/right modified Blalock–Taussig shunt; PA=pulmonary atresia; PFAA=persistent fifth aortic arch; PGE1=prostaglandin E1; SPCA=systemic–pulmonary collateral artery; TTE=transthoracic echocardiography; VSD=ventricular septal defect
Discussion
The true embryological origins of the structures described as persistent fifth aortic arch remain controversial. Indeed, despite their anatomical similarities, it has been suggested that the two major subtypes classified by Freedom may in fact have separate embryological origins. Such limited data exist for the remaining subtypes that their very existence has been called into question.Reference Gupta, Gulati and Anderson 13
It is conventionally accepted that mammals develop six paired pharyngeal arches, which do not co-exist, but variously involute to form the aortic arch and head and neck vessels. The left fourth arch is said to form the definitive (left-sided) aortic arch, with the developing pulmonary arteries originating from the mid-ventral portions of the sixth arches. The distal portion of the left sixth arch also forms the arterial duct.Reference Anderson, Chaudhry and Mohun 42 The fifth arches, by contrast, are thought to be either absent in humans, or transient structures that leave no remnant in the definitive arch system. Embryological data on the existence of such structures in the normal mammalian fetus are scarce. An arterial structure has been detected – in just a single human fetus – within the pharyngeal mesenchyme extending almost the entire distance between the aortic sac and the distal aorta. Thus, although this finding offers the strongest embryological explanation for persistent fifth aortic arch, it could only endorse those forms with a “systemic-to-pulmonary” connection.Reference Bamforth, Chaudhry and Bennett 1 , Reference Lorandeau, Hakkinen and Moore 43 By contrast, separate dorsal collateral channels have been identified in up to one in eight normal human embryos; however, in all cases these vessels communicated directly between the fourth and sixth arches, as opposed to arising from the aortic sac.Reference Bamforth, Chaudhry and Bennett 1 Therefore, while potentially accounting for the “systemic-to-systemic” form of persistent fifth aortic arch, this would also imply that these vessels are not, in fact, true remnants of the fifth pharyngeal arch.
The majority of the clinical literature describes isolated case reports, and, although the provenance of many of these descriptions is debatable,Reference Gupta, Gulati and Anderson 13 there remain long-standing concerns that these types of abnormalities remain under-recognised.Reference Gerlis, Ho, Anderson and Da Costa 11 This may be due to the striking similarities to other more common congenital vascular malformations; however, there are many important reasons beyond a passing academic interest for considering the diagnosis. Systemic-to-systemic connections may form a double aortic arch that does not produce a vascular ring, or provide a critical distal aortic connection in severe arch abnormalities, which allows them to remain undiagnosed well into adulthood, with implications to both the method of intervention and longer-term follow-up. A systemic-to-pulmonary persistent fifth aortic arch can lead to symptomatic left-to-right shunting in neonates and infants, or be a vital aortopulmonary connection in severe right-sided obstructive lesions. Each type has been associated with early and late coarctation, and may have an unpredictable response to intravenous prostaglandin in the neonatal period.
A further comprehensive review by Gupta et al in 2014Reference Gupta, Bamforth and Anderson 44 also draws our attention to the fact that a channel qualifying as a fifth aortic arch has only ever been found in one human embryo, and this occupied only a relatively discrete portion of the pharyngeal mesenchyme. This review lists all published cases according to either the double-barrelled form or aortic to pulmonary artery connection, and concludes that alternative embryological explanations for the vast majority of structures labelled as fifth aortic arches may be more likely. Nevertheless, the cases in this article demonstrate a number of aortic arch abnormalities of undoubted clinical significance that are difficult to explain as either arterial ducts or aortopulmonary windows.
Conclusion
The embryological derivation of the so-called “persistent fifth aortic arch” in humans remains controversial; as our understanding of its true origins continues to improve, a more accurate definition is likely to evolve. In clinical practice, however, this group of abnormalities are almost certainly under-recognised, with potentially important consequences. Increasing awareness, coupled with increasing use of advanced cross-sectional imaging techniques, continues to provide important insights into the true nature and prevalence of these unusual structures.
Acknowledgements
The authors thank Dr Michael Rubens, Consultant Cardiothoracic Radiologist, Royal Brompton Hospital, for his imaging expertise. The authors also thank Dr Nick Archer, Paediatric Cardiology Department, John Radcliffe Hospital, Oxford, for his support.
Financial Support
This project was supported by the NIHR Cardiovascular Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London.
Conflicts of Interest
None.