Pericardiocentesis is an invasive percutaneous procedure for acute and chronic excessive accumulation of pericardial fluid. There are a wide range of etiologies for pericardial effusion, such as idiopathic, malignant, infectious, autoimmune, cardiac, and post-pericardiotomy syndrome in children. Pericardiocentesis is the one-time needle aspiration of pericardial fluid. It is often accompanied by the placement of a pericardial drain that allows for continuous drainage. In most cases, pericardiocentesis with and without pericardial drain placement suffices to manage a pericardial effusion. However, chronic and recurrent pericardial effusion can occur, requiring repeat pericardiocentesis and/or creation of a pericardial window. The most serious complication associated with pericardiocentesis is hemopericardium caused by inadvertent myocardial puncture. The effectiveness and safety of pericardiocentesis in children has not been reported well in the literature and primarily only small studies. Reference El-Najdawi, O’Leary and Seward1–Reference Lock, Bass, Kulik and Fuhrman6 We hypothesised that pericardiocentesis with and without pericardial drain placement is effective and safe in children and the risk of complication is associated with certain clinical factors. The objectives of this study were to describe our single-centered 20-year experience of pericardiocentesis in children at a tertiary children’s hospital, to evaluate the effectiveness and safety of pericardiocentesis, to evaluate factors associated with acute procedural failure and development of adverse events, and to describe a practice pattern in using concurrent drain placement over the study period.
Methods and materials
This was a retrospective study to describe all of the patients who underwent pericardiocentesis with and without pericardial drain placement at the bedside or in the pediatric cardiac catheterisation laboratories for pericardial fluid accumulation at the Children’s Hospital of Michigan. The study period was 20 years (2001–2020). The cardiac catheterisation and echocardiography database were used to identify eligible patients aged ≤20 years of age. Patients aged 21 years or above were excluded. This study was approved by the Institutional Review Board at Wayne State University. Data on demographics, clinical history, physical findings, surgical history, laboratory tests, electrocardiography, echocardiography, cross-sectional imaging, cardiac catheterization, and clinical follow-up were collected from the medical records. The etiologies of pericardial effusion were categorised into idiopathic, malignant, autoimmune, infectious, iatrogenic, cardiac, nephrogenic, and post-pericardiotomy syndrome. Types of underlying cardiac diagnosis and previous/concurrent cardiac surgeries were documented. The hemodynamic impact of pericardial effusion was categorised into none, echocardiographic tamponade, and clinical tamponade. Echocardiographic tamponade was defined as echocardiographic findings of right ventricle or atrial collapse, a >30% change in the mitral inflow Doppler signal with inspiration and expiration without evidence of clinical tamponade. Reference Fowler7 Clinical tamponade was defined as clinical features of hypotension, pulsus paradoxus, increased central venous pressure if invasive venous line present with Kussmal’s sign, or decreased cardiac output. Reference Bilchick and Wise8 The indications (therapeutic versus diagnostic) of pericardiocentesis were determined along with location of procedures (bedside versus catheterisation laboratory) and acuity of procedures (emergent and non-emergent). Emergent was defined as the patient being critically unstable requiring the pericardiocentesis to be done immediately due to concern for imminent cardiac arrest or patient already having arrested due to pericardial effusion. Echocardiographic findings included the size (small, moderate, and large) and distribution (circumferential and posterior). Concurrent medical therapies for pericardial effusion were collected: Nonsteroidal anti-inflammatory drugs (NSAIDs); colchicine; steroids; and their duration. Acute procedural failure was defined as pericardiocentesis with non-satisfactory reduction of pericardial fluid shown on echocardiography and/or one which was aborted due to significant complications. After the first pericardiocentesis for the same illness, the data was collected on the need of repeat pericardiocentesis. The timing to remove the pericardial drain was at the discretion of individual providers. Typically, the drain was removed when echocardiography confirmed no evidence of re-accumulation of pericardial fluid after clamping the drain for 24 hours. Adverse events were categorised into major and minor based on the severity of adverse events. Major adverse events included death, hemopericardium requiring emergent surgery, resuscitation, and significant hypotension. Minor adverse events included inadvertent cardiac puncture without need of further intervention and other complications without negative consequences.
Pericardiocentesis
Pericardiocentesis was performed either at the bedside or in the cardiac catheterisation laboratory. General anesthesia or moderate sedation was administered by anesthesiologists or pediatric intensivists. Patients were often placed in a semi-reclining position and the majority of pericardiocentesis were performed via a subxiphoid approach. Under transthoracic echocardiographic guidance, a micropuncture needle was advanced into the pericardial space. When a pericardial drain was placed, a 3–8-Fr Pigtail catheter was positioned in the pericardial space and secured by sutures at the skin. When the procedure was performed in the cardiac catheterisation laboratory, fluoroscopy was used to confirm positioning of the needle, wire, and/or pericardial drain. Transthoracic echocardiography confirmed the effective aspiration of pericardial fluid at the end of the pericardiocentesis. The aspirated volume of pericardial fluid was classified into small (<5 ml/kg), moderate (5–15 ml/kg), and large (>15 ml/kg).
Statistical analysis
Data was expressed as median with range and number with percent. Chi-square or Fisher’s exact test was used to compare the categorical variables between two groups. A p < 0.05 was considered statistically significant. Statistical analysis was performed using the SPSS version 26 (SPSS, IBM Inc., Armonk, NY, USA).
Result
Demographics and etiologies (Table 1)
Table 1. Patient demographics, underlying diagnoses, and clinical presentation for the initial pericardiocentesis in 127 patients

Data are expressed as number (percent) or median (range)
Our study cohort consisted of 153 total pericardiocentesis in 127 patients. Repeat interventions were performed in 33 patients. The median age was 6.5 years (1 day–20 years) with the most frequent age group being adolescents (n = 59, 47%). The median weight was 17 kg (range 0.5–125 kg).

Figure 1. Etiologies of pericardial effusion in 127 children.
The most common etiology (Fig 1) was post-pericardiotomy syndrome (n = 56, 44%), followed by infectious (n = 15, 12%) and malignant (n = 13, 10%). The most common infectious etiology was viral (n = 5), followed by staph aureus (n = 3). Nearly half of patients (n = 60, 47%) had underlying congenital heart diseases (Supplemental Table 1). Among the 56 patients with post-pericardiotomy syndrome, the most common preceding cardiac surgery was atrial septal defect/ventricular septal defect repair (n = 9, 16%), followed by heart transplant (n = 7, 13%), and mitral valve repair/replacement (n = 7, 13%). For these patients, the median duration between surgery and pericardiocentesis was 21 days (range 4–75 days).
Clinical characteristics
Clinical tamponade was seen in 32 patients (25%), echocardiographic tamponade 45 patients (35%), whereas 50 patients (39%) did not exhibit cardiac tamponade signs or symptoms. Most patients (n = 75, 65%) were on room air and the remaining were on respiratory support such as mechanical ventilation (n = 30), Bilevel positive airway pressure (BiPAP) (n = 1), and high flow nasal cannula oxygen (n = 10). The size of pericardial effusion based on echocardiography was classified into large (n = 70), moderate (n = 55), and small (n = 1). Prior to the pericardiocentesis, most patients were treated medically with steroids (n = 38), NSAIDs (n = 17), and colchicine (n = 4) and 46 patients did not have any preceding medical therapy.
Pericardiocentesis (Table 2)
Table 2. Procedural details of pericardiocentesis (n = 153)

Among 153 pericardiocentesis, concurrent pericardial drain placement was performed in 67 patients (53%). The majority of the procedures were performed in the cardiac catheterisation laboratory (n = 88, 59%), while the remainder were performed in either the pediatric intensive care unit (n = 48, 32%), neonatal intensive care unit (n = 12, 8%), or the post-anesthesia care unit (n = 1, 1%). Pericardiocentesis was performed emergently in 14 instances (9%). Subxyphoid approach was used in the majority (n = 107, 70%). Over the study period, procedures were more frequently performed in the cardiac catheterisation lab versus bedside (Fig 2).

Figure 2. Yearly trend of pericardiocentesis frequency based on ( a ) the location and ( b ) the use of concurrent pericardial drain placement.
Effectiveness (Table 3)
Table 3. Effectiveness, safety, and outcome of pericardiocentesis (n = 153)
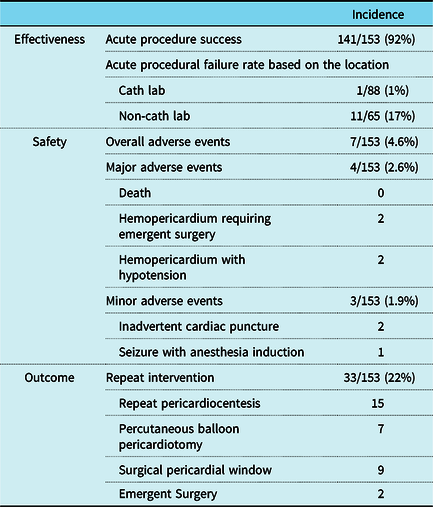
Pericardiocentesis was acutely successful in 92% of procedures (n = 141) with small or less pericardial effusion remaining post procedure and no complication requiring emergent surgery. Pericardiocentesis at the bedside had a higher rate of acute procedural failure than that in the catheterisation lab (17 versus 1%, p < 0.01). Acute procedural failure cases (Table 4) were seen in either small infants (<5 kg) or older larger patients (>30 kg). A median of 9 ml/kg (0–28ml/kg) of pericardial fluid was removed during the procedure. The volume of fluid removed is further broken down as small (24%), moderate (56%), and large (17%) (Table 2). Concurrent pericardial drain placement was done in 66 patients. The average length of time the drains were left in place was 4 days (1–19 days). Patients were more likely to have a pericardial drainage catheter placed if the procedure was performed in the catheterisation lab (46% bedside versus 63% catheterisation lab) though this difference was not found to be statistically significant.
Table 4. Cases of acute procedural failure (n = 12)

NICU, neonatal intensive care unit; PACU, post-anesthesia care unit. PICU, pediatric intensive care unit; PPS, post-pericardiotomy syndrome
After initial pericardiocentesis and/or pericardial drain placement 51% (n = 62) of patients had re-accumulation of pericardial effusion. Of these patients, 33 patients (22%) required re-intervention (Fig 3). The median time between interventions was 12 days (0–129 days). There was no difference in the rate of re-intervention with and without concurrent drain placement (24 versus 22%, p = 0.811). However, in the subset of patients with post-pericardiotomy syndrome, the use of concurrent drain placement appears to have a lower re-intervention rate (29 versus 50%) but the difference was not statistically significant (p = 0.163).
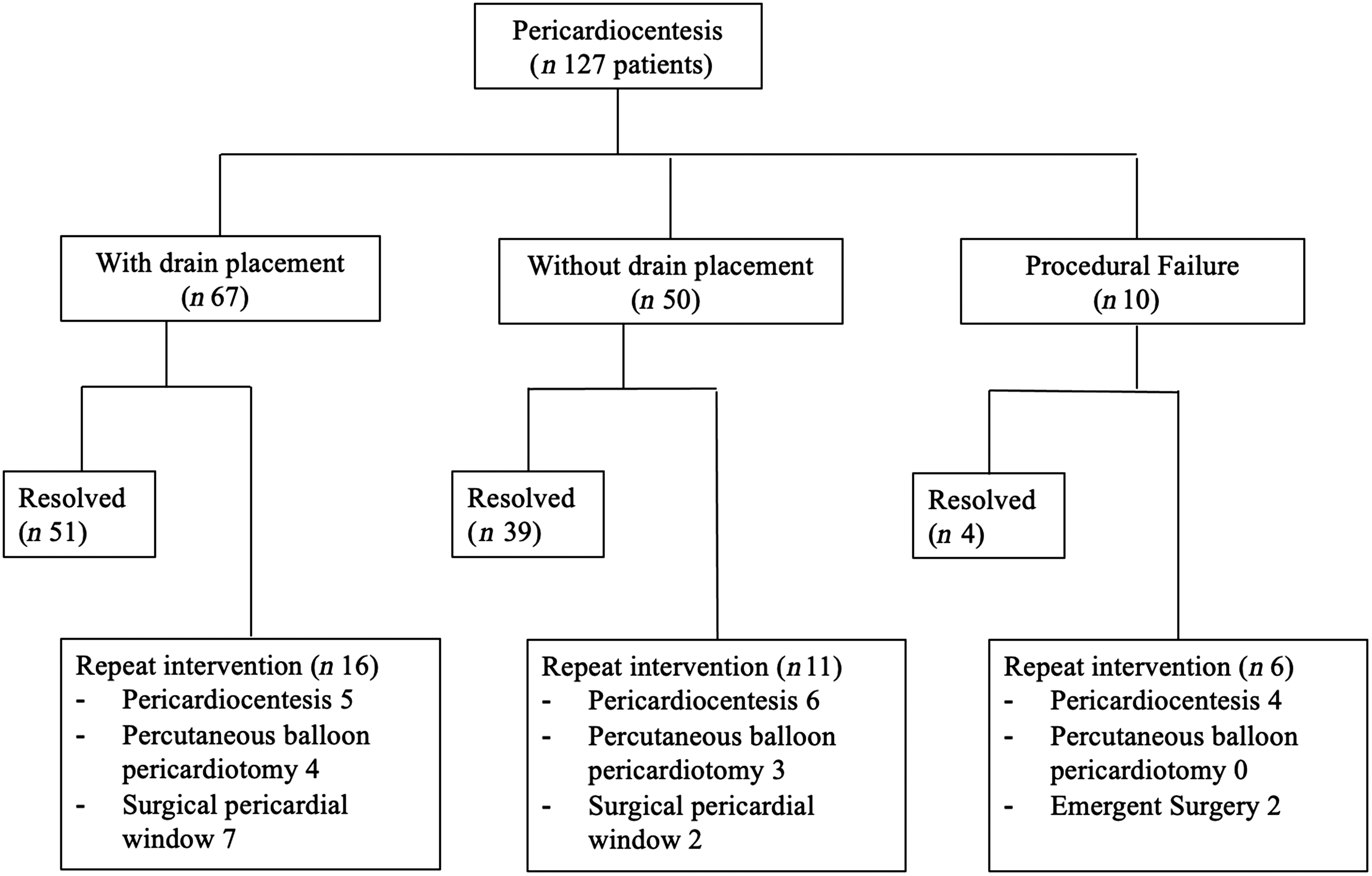
Figure 3. Flow chart of 127 patients undergoing pericardiocentesis with and without concurrent pericardial drain placement.
Safety
The overall incidence of adverse events was 4.6% (7/153). Major adverse events (n = 4) were hemopericardium requiring emergent surgery (n = 2), of which one had cardiac arrest requiring cardiopulmonary resuscitation, and hemopericardium with hypotension (n = 2). Minor adverse events (n = 3) were seizure with anesthesia induction (n = 1) and inadvertent right ventricle puncture (n = 2). There were no clinical factors associated with the development of adverse events: age; weight, etiology; amount of pericardial effusion; repeat pericardiocentesis; cardiac tamponade; location and urgency of procedure; and pericardiocentesis approach (Supplemental Table 2).
Discussion
This study shows the safety and effectiveness of pericardiocentesis in children. This is one of the largest studies evaluating the immediate outcomes of pericardiocentesis in a pediatric population. In our cohort, the overall adverse event rate was 4.6%. This incidence is similar to the reported complication rates seen in the adult population. Reference Maisch, Ristic, Pankuwiet and Seferovic9 Acute procedural success rate was 92%, though 22% of patients did require re-intervention. Our study showed that pericardiocentesis can be safely performed at either the bedside or catheterisation lab though pericardiocentesis was much more successful when performed in the catheterisation lab. Emergent pericardiocentesis was life-saving without an increase of adverse events.
A large single center adult review of pericardiocentesis over 21 years was reported by Tsang et al. Reference Tsang, Enriquez-Sarano and Freeman10 The overall complication rate was 4.7% with 1.2% being major events requiring further intervention. Our study demonstrated a similar finding with this adult study. Previously reported complication rates in children were 4–14%. Reference Law, Borasino, Kalra and Alten2–Reference Tsang, El-Najdawi, Seward, Hagler, Freeman and O’Leary4 In our study, the most significant adverse events were hemopericardium in four cases, of which two required emergent surgery. When hemopericardium occurs due to cardiac puncture, an intrapericardial thrombus is often difficult to be evacuated by percutaneous drainage. Although irrigation of the intrapericardial space with heparin may be attempted to dissolve the freshly formed thrombus, emergent surgical intervention should be strongly considered for uncontrolled hemopericardium.
The most frequent etiology of pericardial effusion requiring pericardiocentesis was post-pericardiotomy syndrome (44%) in our cohort. In the study of pediatric post-pericardiotomy syndrome, Reference Elias, Glatz and O’Connor11 the re-admission rate due to pericardial effusion was 1.1%, of which 44% required intervention. Consistent with this previous report, cardiac surgeries associated with post-pericardiotomy syndrome and the need for intervention were atrial septal defect repair, ventricular septal defect repair, and heart transplant in our cohort.
Our center had a larger percentage of procedures performed in the cardiac catheterisation laboratory with the use of concurrent pericardial drain placement though this difference as stated previously did not reach statistical significance. This is partly because fluoroscopy allows operators to evaluate the wire and catheter course as well as the pericardial drain placement becoming more straightforward. In our practice, the catheterisation laboratory has become the preferred location for pericardiocentesis because of fluoroscopic guidance capability and sterile procedural environment. The downside of its use is the increase of resource utilisation, expense, and need to transfer patients to the laboratory. Previous reports showed that pericardiocentesis can be safely performed at the bedside. Reference Law, Borasino, Kalra and Alten2,Reference Drummond, Seward, Tsang, Hayes and Miller12 In our study, there was no difference of adverse event rates between in the catheterisation laboratory and at the bedside. However, there were more cases of acute procedural failure at the bedside, especially in small infants or larger patients. We speculate that fluoroscopy in addition to echocardiography may be helpful to guide pericardiocentesis. This is the reason why our program uses the catheterisation laboratory as the preferred place for pericardiocentesis.
Since the concurrent pericardial drain placement is our preferred method currently, the effect of drain placement on re-intervention was evaluated. Although there was no difference in the overall cohort, the need for re-intervention was 29% with the use of pericardial drain compared to 50% without it in the subsets of post-pericardiotomy syndrome. Unfortunately, this difference was not statistically significant due to a small sample size. The use of pericardial drain has been evaluated by two previous studies. The recurrence rate of cardiac tamponade significantly dropped with the use of an extended use of pericardial drain (12 versus 52%). Reference Rafique, Patel and Biner13 Another study showed the use of a pericardial drain decreased the recurrence rate of pericardial effusion from 27 to 14%. Reference Tsang, Enriquez-Sarano and Freeman10 A larger study may be considered to evaluate the effect of a pericardial drain in children.
Surgical pericardial window creation is a final resort for recurrent pericardial effusions. As shown in our study, almost all of the patients are treated with pericardiocentesis and surgical intervention is performed in refractory cases. In our cohort, there were 10 patients (5.9%) requiring surgical pericardial window. Previous literature has shown the high success rate of surgical pericardial window in the adult population. Reference Langdon, Seery and Kulik14–Reference Saltzman, Paz and Rene17 The other consideration for refractory and recurrent pericardial effusion is percutaneous balloon pericardiotomy, which creates a window in the parietal pericardium by balloon dilation. Our experience has been reported elsewhere with high technical success rate (100%) with no acute adverse events. Reference Herron, Forbes and Kobayashi18
Limitation
This was a retrospective study with an inherent limitation. The study was conducted in a single center and the sample size was not large, limiting the generalizability. The study cohort had heterogeneous etiologies and various providers performed pericardiocentesis over 20 years. There is a possibility of under-reporting complications due to lack of detailed documentation, although all the available old paper-based medical records were reviewed. However, the major adverse events should have been captured because of the need of interventions. A major confounding factor was concurrent medical therapy before and after pericardiocentesis, that would have a potential effect on the recurrent pericardial effusion.
Conclusion
Pericardiocentesis was life-saving in children with its high effectiveness and safety even in urgent situations. Although initial pericardiocentesis was effective, one in five patients required re-intervention for recurrent pericardial effusion. In the subset of post-pericardiotomy syndrome, concurrent drain placement may be helpful to avoid re-intervention. Pericardiocentesis was more frequently successful if performed in the cardiac catheterisation laboratory.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S104795112100278X.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
All procedures performed in studies involving patients were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all patients included in this case report.










