Coronary sinus atrial septal defects are the rarest defects of the atrial septum comprising <1% of the five different types of atrial septal defects.Reference Joffe, Rivo and Oxorn 1 Despite the widespread adoption of percutaneous device closure of secundum atrial septal defects, uncertainty regarding the ability to safely and effectively close coronary sinus atrial septal defects remains. The frequent presence of a persistent left superior caval vein adds an additional level of complexity. The published experience with percutaneous transcatheter device closure of coronary sinus atrial septal defects is limited to only a few isolated case reports. For these reasons, currently, open-heart surgery remains the treatment of choice for coronary sinus atrial septal defects.
Since the first successful percutaneous transcatheter closure of a coronary sinus atrial septal defect reported in 2003,Reference Di Bernardo, Fasnacht and Berger 2 infrequently used and differing percutaneous methods to treat coronary sinus atrial septal defects have been described in a small number of case reports.Reference Di Bernardo, Fasnacht and Berger 2 – Reference Torres, Gersony and Hellenbrand 5 We describe here our own experience with two adult patients with different presentations and our method of successful percutaneous coronary sinus atrial septal defect closure in each. We then present a review of the anatomic spectrum of coronary sinus atrial septal defects along with the review of contemporary surgical and percutaneous device treatment.
Case presentations
Case 1
An 18-year-old woman was referred to our cardiac catheterisation laboratory for evaluation of an isolated coronary sinus atrial septal defect. In 2004, at the age of 9, she had an echocardiogram for evaluation of a murmur, which was interpreted as showing a 4 mm coronary sinus versus primum septal defect and a mildly dilated right ventricle. She underwent cardiac magnetic resonance imaging the following year that could not distinguish between a primum or coronary sinus defect because of motion artefact. Magnetic resonance imaging estimated the size of the defect as 7 mm, with an estimated Qp:Qs of 1.2. Mild right ventricular dilatation was also noted. The patient’s parents declined transcatheter or surgical treatment at the time. After several years of observation, in 2007, the patient’s parents agreed to a cardiac catheterisation. This demonstrated a coronary sinus-type atrial septal defect. Surprisingly, there was no left-to-right shunt detected by oximetry and the pulmonary artery pressures were normal. Mild-to-moderate right ventricular dilatation was confirmed and thought to be out of proportion to the size of the coronary sinus septal defect. The patient underwent repeat cardiac magnetic resonance imaging to rule out arrhythmogenic right ventricular cardiomyopathy or some other myopathy. The study did not visualise the coronary sinus septal defect again because of motion artefact and was not diagnostic of right ventricular myopathy or arrhythmogenic right ventricular cardiomyopathy. Throughout this period, the patient remained asymptomatic with no apparent limitations to her activity level. By age of 17 years, however, she began to report symptoms of chest discomfort and shortness of breath. She was referred for a third cardiac magnetic resonance imaging study, which now showed the defect size at 8–10 mm with an increase in Qp:Qs to 1.5. At this point, the patient and her parents agreed to a second catheterisation to assess the feasibility of percutaneous device closure.
Right heart pressures were similar to those measured when the patient was 10 years old. The right ventricular end diastolic pressure was slightly higher at 12 mmHg. The Fick measured Qp:Qs ratio had increased to 1.8:1. Intracardiac echocardiography characterised the defect as a coronary sinus atrial septal defect with extension to the secundum septum superiorly (Fig 1; Kirklin and Barratt-Boyes; Type IV). The total elliptical-shaped defect diameter appeared to be about 8×10 mm, and was relatively close to the ostium of the coronary sinus. An end-hole catheter and hydrophilic guidewire were used to position an 0.035’ Amplatzer Super Stiff ST1 (Boston Scientific, Washington, District of Columbia, United States of America) interventional wire through the coronary sinus ostium, through the terminally unroofed region, and into the left upper pulmonary vein. A 24 mm sizing balloon yielded a stop flow measurement of 13.5–14 mm (Fig 2). A 14 mm Amplatzer® Septal Occluder device (St. Jude Medical, Saint Paul, Minnesota, United States of America) was deployed through a 7 Fr TorqView® delivery sheath (St. Jude Medical) and positioned such that the left atrial disc was within the left atrium forming the front wall of the coronary sinus – missing or “unroofed” portion. The waist of the device was then filling the defect and extending into the secundum septum superior to the coronary sinus ostium with the right atrial disc anchored against the atrial septum inside the right atrium (Fig 3). With the device still tethered to its delivery cable, intracardiac echocardiography and coronary angiography with venous phase follow-through were performed. There was no obstruction in the coronary sinus flow, no interference with the mitral valve leaflet function, and no change in the cardiac conduction rhythm or new-onset ectopy. The device was then successfully released from its delivery cable and repeat coronary arteriograms were repeated again with satisfactory results. Outpatient follow-up 2 weeks after device implant demonstrated the patient to be asymptomatic, and an echocardiogram demonstrated the device in good position, with no residual shunt through the Amplatzer® Septal Occluder device and trivial mitral insufficiency.
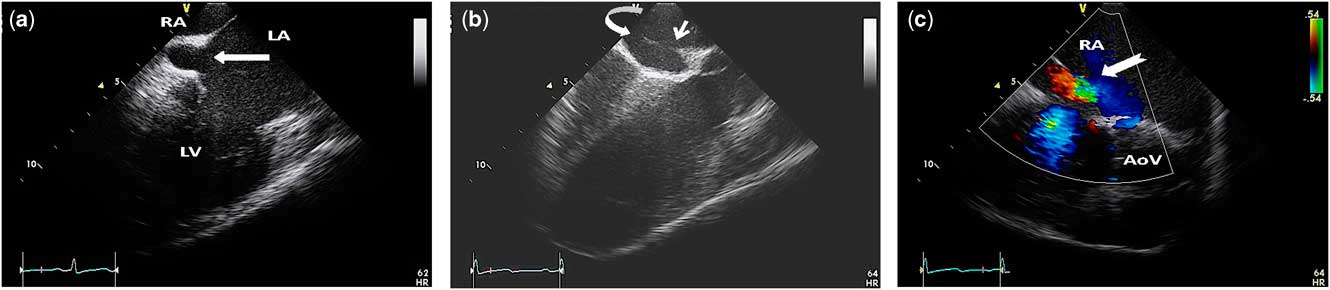
Figure 1 (a) Intracardiac echocardiography imaging demonstrating “unroofing” of a portion of the front wall of the coronary sinus – solid arrow – thus creating a coronary sinus atrial septal defect. (b) An off-axis intracardiac echocardiography image of the secundum atrial septum immediately superior to the coronary sinus ostium – curved white arrow – showing the extension of the coronary sinus defect into the adjacent secundum atrial septum – thin white arrow. (c) A colour Doppler intracardiac echocardiography image demonstrating turbulent left-to-right flow through the atrial septal defect – tagged white arrow. AoV=aortic valve; LA=left atrium; LV=left ventricle; RA=right atrium.

Figure 2 (a) Intracardiac echocardiography image showing a catheter – solid white arrow – advanced from the right atrium through the secundum atrial septal defect, along the course of the coronary sinus, through the defect into the left atrium. The mitral valve is visible immediately below the level of the coronary sinus – hollow white arrow. (b) Balloon sizing showing the diameter of the balloon when the point of cessation of shunting was reached – balloon stop flow diameter. (c) Corresponding fluoroscopic image taken at balloon stop flow showing the atypical position of the sizing balloon.
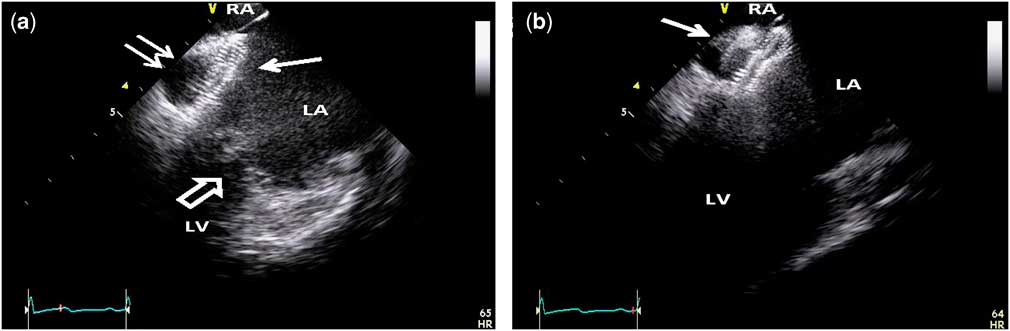
Figure 3 (a) Intracardiac echocardiography image showing the left atrial disc – single solid white arrow – of the septal occluder tightly adherent to the front wall of the coronary sinus – double arrow. Also, note that the left atrial disc is above and not interfering with the adjacent anterior mitral valve leaflet – hollow white arrow – excursion. The inferior atrial septum is just visible at the top of the image sector. (b) Intracardiac echocardiography image angled slightly superior showing the waist of the occluder device traversing the coronary sinus defect and extending through the septum secundum with the proximal disc – white arrow – within the right atrium. LA=left atrium; LV=left ventricle; RA=right atrium.
Case 2
A 46-year-old woman had a past medical history of breast cancer, hypothyroidism, and developed a right superior caval vein thrombosis from a Port-A-Cath – long-term central line – placed for administration of chemotherapy. She was diagnosed with breast cancer in 2001 and after undergoing a bilateral mastectomy, she underwent Port-A-Cath placement in May of 2011 to facilitate her chemotherapy. Later that year, she experienced a transient ischaemic attack manifested by a sudden onset slurred speech and a change in mental status. She was started on aspirin and clopidogrel, and an echocardiogram by report revealed a thrombus in close proximity to the distal Port-A-Cath as well as an apparent patent foramen ovale. Percutaneous device closure of her patent foramen ovale was performed at an outside institution in June 2011 using an Amplatzer® Cribriform Atrial Septal Occluder. Approximately 10 months later, her transient ischaemic attack symptoms recurred, and in May 2012, she was taken to the operating room at the same outside institution after an echocardiogram revealed a persistently positive micro-bubble contrast study. With the patient on cardio-pulmonary bypass, the right atrium was opened and the septum inspected. The septal occluder device was found in good position in the area of the superior fossa ovalis; however, a defect was noted in the lower atrial septum extending into the coronary sinus (Kirklin and Barratt-Boyes; Type IV) where the terminal roof of the coronary sinus was absent. The previously placed patent foramen ovale device was removed and she underwent stitch closure of her patent foramen ovale and patch closure of her coronary sinus atrial septal defect. Over the next several weeks, she remained symptom free and her anticoagulation regimen was discontinued. However, 2 months later, she began developing dyspnoea with exertion, easy fatigability, and occasional cyanosis of her hands and feet with activity. She was referred to our institution for the first time in April 2013 for further evaluation. Additional magnetic resonance imaging and echocardiography demonstrated a persistent left superior caval vein to coronary sinus. Micro-bubble contrast injection into a left arm peripheral intravenous line demonstrated micro-bubbles in the left atrium almost immediately. Further imaging suggested a partial coronary sinus atrial septal defect patch dehiscence with functional recurrence of her coronary sinus atrial septal defect. She was referred for cardiac catheterisation for possible percutaneous closure of her residual coronary sinus atrial septal defect.
Right heart catheterisation was performed under general anaesthesia. Right heart pressures were normal and no net shunting was detected by oximetry. Left innominate venography confirmed the presence of both a right and a left superior caval vein with a normal calibre bridging vein between the two. Using a 5-Fr angled Glide catheter with an angled Glidewire, the catheter was advanced from the inferior caval vein into the innominate vein, down the left superior caval vein, and then into the coronary sinus. Multiple contrast injections and intracardiac echocardiography were used to confirm the position of the unroofed coronary sinus and the relative location of the major coronary veins (Fig 4). A bubble saline injection through a left upper extremity vein confirmed right to left shunting into the left atrium. A Glidewire was used to guide a Supercross micro-catheter (Vascular Solutions, Minneapolis, Minnesota, United States of America) from the left superior caval vein through the recurrent coronary sinus atrial septal defect and into the left lower pulmonary vein. The Glidewire was exchanged for a V-18 control wire, which was parked in the left lower pulmonary vein. Over this wire, the Supercross catheter was exchanged for a valvuloplasty balloon, used for sizing the atrial septal defect. The balloon stop flow diameter was measured at 5 mm (Fig 5). After exchanging up to a 0.035 guidewire, and advancing a 7 Fr TorqView® Delivery sheath across the defect, a 7 mm Amplatzer® Septal Occluder was implanted under intracardiac echocardiography guidance. Using a similar technique to that used in the case described above, the device was implanted into the defect. Before releasing the device, angiography of the left superior caval vein confirmed appropriate placement of the device without obstruction to coronary sinus flow or distortion of the coronary venous anatomy. In addition, a significant right superior caval vein stenosis, which was also identified during the catheterisation, was then stented with an excellent angiographic appearance result achieved with an increase in the minimal luminal diameter of the affected portion of the superior caval vein from 5.8 to 20 mm. Before concluding the case, right and left coronary angiography with venous phase follow-through was performed demonstrating no obstruction in coronary sinus flow. A final injection into the left superior caval vein demonstrated the septal occluder device in good position, no significant residual left-to-right shunting, and no apparent obstruction to coronary sinus drainage into the right atrium (Fig 6). Two-week follow-up demonstrated no residual shunt through the Amplatzer® Septal Occluder device and was otherwise normal. The patient was in normal sinus rhythm. The patient’s symptoms improved including cessation of her multiple times per week transient ischaemic attacks.
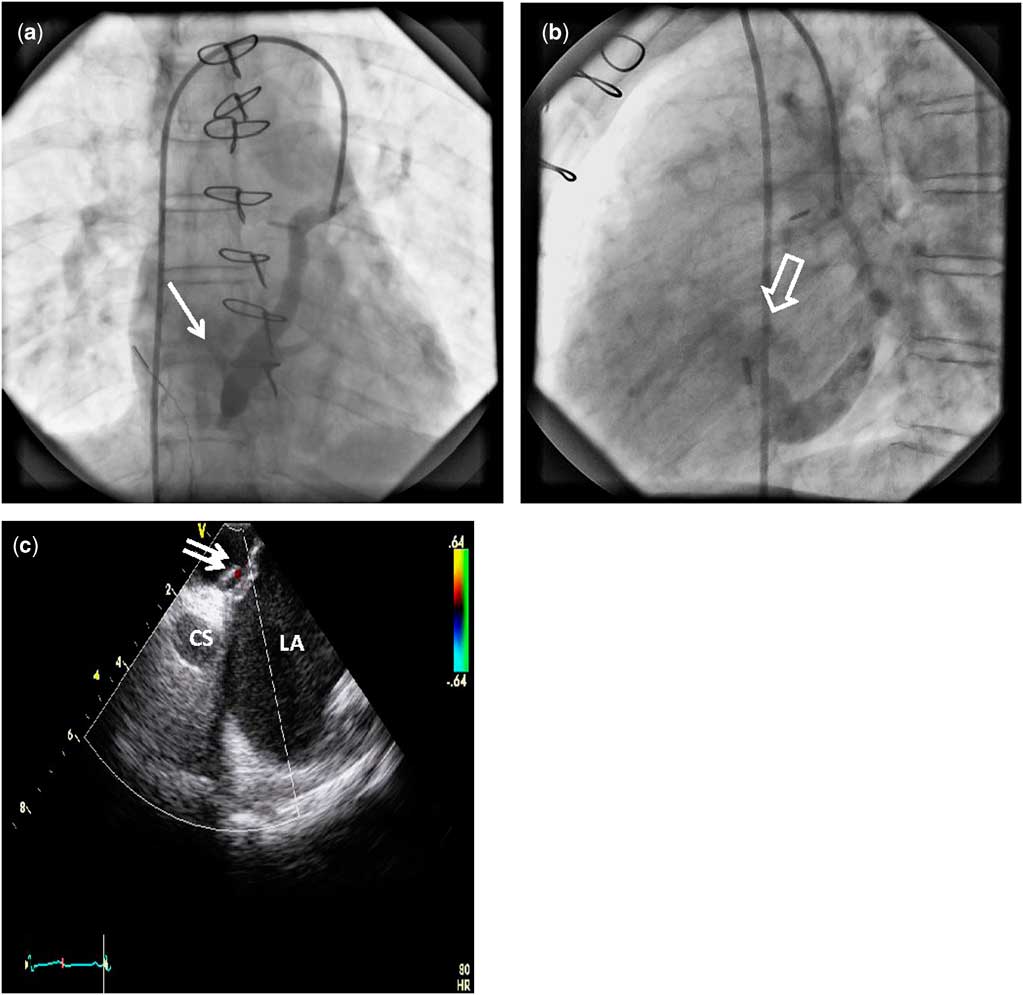
Figure 4 (a) Angiogram performed in mid-left superior caval vein showing contrast filling the coronary sinus. Some contrast is also seen streaming into the left atrium – white arrow. (b) Companion lateral angiographic image to Figure 4a showing the coronary sinus draining into the right atrium; however, a portion of the column of contrast is also seen coursing posteriorly into the left atrium – white block arrow – immediately above the ostium of the coronary sinus. (c) Intracardiac echocardiography image with colour Doppler showing shunting around a portion of the surgical patch, which is non-adherent and lifted off the plane of the atrial septum – double white arrow. CS=coronary sinus; LA=left atrium.

Figure 5 (a) Intracardiac echocardiography image taken during balloon sizing of the defect showing the stop flow diameter of 5 mm – white arrow. (b) Corresponding fluoroscopic image showing position of the sizing as well as the atypical guidewire course – see text. Balloon measurement at balloon stop flow was ∼5.6 mm.

Figure 6 A final left superior caval vein angiogram taken in the straight anterior–posterior projection following occlusion of the residual coronary sinus atrial septal defect and stent angioplasty of a long segment right superior caval vein stenosis related to previous radiation therapy for breast cancer. The Amplatzer® Septal Occluder is visible in the residual coronary sinus atrial septal defect – solid white arrow. An injection of contrast into the innominate vein shows contrast flowing into the coronary sinus and draining into the right atrium – block white arrow.
Review and discussion
Anatomic spectrum and pathophysiology
The coronary sinus itself is formed from the left horn of the sinus venosus during cardiac embryology at the same time that the right horn of the sinus venosus is being incorporated into the right atrium.Reference Moss and Allen 6 In addition, this occurs near the same time that the paired anterior and posterior cardinal veins are involuting on the left and persisting on the right to form the normal right-sided superior caval vein and proximal inferior caval vein.Reference Moss and Allen 6 Interatrial communications at the expected site of the coronary sinus are rare and may be isolated but often occur together with persistence of a left superior caval vein.Reference Ootaki, Yamaguchi, Yoshimura, Oka, Yoshida and Hasegawa 7 , Reference Raghib, Ruttenberg, Anderson, Amplatz, Adams and Edwards 8 Such defects are usually located anteriorly and inferiorly to the fossa ovalis. The developmental entity of a persistent left superior caval vein, the absence of a separating septum between the coronary sinus and left atrium – that is, complete “unroofing” of the coronary sinus – and the presence of an atrial septal defect in the region normally occupied by the coronary sinus ostium was first recognised and described by Raghib et alReference Raghib, Ruttenberg, Anderson, Amplatz, Adams and Edwards 8 in 1965 and today carries the name of Raghib’s syndrome.
Unroofed coronary sinus have been further subclassified into four groups by Kirklin and Barratt-Boyes: type I, completely unroofed with the left superior caval vein; type II, completely unroofed without the left superior caval vein; type III, partially unroofed midportion; and type IV, partially unroofed terminal portion.Reference Ootaki, Yamaguchi, Yoshimura, Oka, Yoshida and Hasegawa 7 Defects in the latter two categories may or may not have an associated left superior caval vein. The diagnosis of this lesion is important for overall patient prognosis because any degree of unroofing of the coronary sinus results in a left-to-right shunt, leading to pulmonary overcirculation or bidirectional shunt-producing symptoms of dyspnoea, cyanosis, cerebral abscess, and/or transient ischaemic attacks.Reference Ootaki, Yamaguchi, Yoshimura, Oka, Yoshida and Hasegawa 7 , Reference Attenhofer-Jost, Connolly, Danielson, Dearani, Warnes and Jamil Tajik 9
Diagnostic challenges
Given their relative infrequency, anatomic location and variable size, as well as non-specific associated symptoms, the diagnosis of coronary sinus atrial septal defects remains a considerable challenge. Coronary sinus atrial septal defects are sometimes associated with secundum atrial septal defects making their diagnosis even more difficult, once all attention is focused on the secundum defect distracting away from the coronary sinus defect.Reference Akkaya, Vuruskan, Aksoy, Ardic, Kucukosmanoglu and Ozer 10 This was clearly illustrated in one patient who underwent prior surgical repair of a secundum atrial septal defect. Left-to-right shunting, through what was thought to be a residual shunt from prior repair on a follow-up transoesophageal echocardiogram, was later found intraoperatively to be a coronary sinus atrial septal defect, thereby indicating that it was missed during the previous surgery.Reference El-Eshmawi, Tang, Pawale, Anyanwu and Adams 11 As described above, this was also the case in our patient – Case 2 – who initially underwent cardiac catheterisation for a suspected defect in the area of the foramen ovale, which was later found to be in fact a coronary sinus atrial septal defect when ongoing neurological symptoms prompted an open-heart surgical exploration.
In one series of 25 patients who underwent surgery from 1958 to 2003 for a multi-fenestrated-type coronary sinus atrial septal defect, Attenhofer-Jost et alReference Attenhofer-Jost, Connolly, Danielson, Dearani, Warnes and Jamil Tajik 9 report that the diagnosis was made before surgery in only 60% of patients, more commonly by pre-operative cardiac catheterisation rather than by pre-operative echocardiography. In the remaining 40%, the diagnosis was made intraoperatively. Similar to the aforementioned case, coronary sinus atrial septal defects were often overlooked during previous cardiovascular surgery in 68% of the patients.Reference Attenhofer-Jost, Connolly, Danielson, Dearani, Warnes and Jamil Tajik 9 , Reference El-Eshmawi, Tang, Pawale, Anyanwu and Adams 11
Transoesophageal and intracardiac echocardiography can provide a superior level of anatomic detail that is very difficult to achieve with standard transthoracic echocardiography, thereby reducing the chances of missing the diagnosis.Reference Bartel and Muller 12 These imaging modalities are now commonly used, resulting in markedly improved diagnostic accuracy compared with transthoracic echocardiography or traditional cardiac catheterisation with angiography.Reference Freedom, Culham and Rowe 13
Experience with primary surgical repair
An understanding of the various combinations of coronary sinus atrial septal defects, with partial/complete unroofing of the coronary sinus with or without a left superior caval vein is necessary for the interventionalist, particularly when there are residual defects following surgical repair – see above for description of Case 2. Ideally, any catheter-based device closure of a coronary sinus atrial septal defect should have the goal of complete closure. An online literature search yielded three surgical series with a combined total of 60 patients with a median follow-up of 7–10 years.Reference Ootaki, Yamaguchi, Yoshimura, Oka, Yoshida and Hasegawa 7 , Reference Attenhofer-Jost, Connolly, Danielson, Dearani, Warnes and Jamil Tajik 9 , Reference Quaegebeur, Kirklin, Pacifico and Bargeron 14 Although the exact details of repair varied among the series based on individual surgical anatomy, the basic strategies in all series consisted of one of the following: patch-roofing the coronary sinus within the left atrium, to direct coronary venous and/or left superior caval vein blood, if present, to the right atrium; closure of the ostium secundum of the coronary sinus to direct drainage to the left atrium. In patients with the addition of a left superior caval vein, Attenhofer-Jost and Ootaki performed test occlusion of the caval vein to decide whether to re-route the caval vein to the right atrium or to ligate the vein completely.Reference Ootaki, Yamaguchi, Yoshimura, Oka, Yoshida and Hasegawa 7 , Reference Attenhofer-Jost, Connolly, Danielson, Dearani, Warnes and Jamil Tajik 9 Mortality rates reported by Attenhofer-Jost, Quagebeuer, and Ootaki were (1/25; 4%), (3/24; 12.5%), and (0/11; 0%), respectively, although most mortalities were encountered in patients who had undergone additional surgeries for associated complex congenital heart disease – for example, heterotaxy, single ventricle anatomy.Reference Ootaki, Yamaguchi, Yoshimura, Oka, Yoshida and Hasegawa 7 , Reference Attenhofer-Jost, Connolly, Danielson, Dearani, Warnes and Jamil Tajik 9 , Reference Quaegebeur, Kirklin, Pacifico and Bargeron 14 No major complications, arrhythmias, or deaths specifically related to the coronary sinus defect were reported in all three surgical cohorts.
Reported percutaneous device closure
Since the first atrial septal defect occlusion device was prototyped by King and Mills in 1974, numerous other devices have come and gone over the years. Currently, in the United States of America, the most widely used devices are the St. Jude Medical Amplatzer® Septal Occluder and the Gore Helex occluder. The Amplatzer® Septal Occluder is a self-centering prosthesis made of nitinol wire mesh with two round disks and a connecting short waist, and was approved by the United States Food and Drug Administration in December 2001. The Gore Helex occluder is also made of a nitinol wire frame covered with an ultra-thin polytetrafluoro–ethylene membrane and was approved by the Food and Drug Administration in 2006.Reference Moore, Hegde and El-Said 15 Both devices are intended for closure of secundum atrial septal defects. With coronary sinus atrial septal defects specifically, the frequent presence of associated anatomic anomalies adds another layer of complexity, making the option of percutaneous closure of the coronary sinus atrial septal defect less desirable than definitive surgical treatment. Currently, there is a paucity of evidence regarding the safety and efficacy of device closure, specifically of coronary sinus atrial septal defects. The published experience has been limited to the following six case reports, which have all used various devices in various anatomic locations for different anatomic combinations of coronary sinus atrial septal defects with unroofed coronary sinus and left superior caval vein – age range from 7 months to 67 years.
Before the Amplatzer® Septal Occluder was approved by the Food and Drug Administration in 2001, left superior caval vein occlusion had been successfully performed without intervening on the coronary sinus itself. In 1997, Heng and DiGiovanni occluded a left superior caval vein draining to an unroofed coronary sinus (Kirklin and Barratt-Boyes; Type 1) with a Gunther Tulip Vein Caval Filter plus vascular coils in a 48-year-old patient.Reference Heng and De Giovanni 16 After two years, Geggel et alReference Geggel, Perry, Blume and Baker 17 reported the case of a 7-month-old infant with a left superior caval vein to left atrium connection and a small defect in the coronary sinus deep within the left atrium. Interestingly, the child had variable cyanosis depending on his head positioning and had no evidence of right heart chamber enlargement. The coronary sinus defect was left alone, and after temporary balloon occlusion of the left superior caval vein produced a desirable increase in systemic saturation with a minimal rise in left superior caval vein pressure, a 9 mm Gianturco–Grifka vascular occlusion sac device was placed within the left superior caval vein.
In 2003, Di Bernardo et alReference Di Bernardo, Fasnacht and Berger 2 in Switzerland were the first to report successful transcatheter closure of a coronary sinus atrial septal defect itself with an Amplatzer® Septal Occluder device in a 9-year-old patient with evidence of right ventricular volume overload. The coronary sinus was partially unroofed in the terminal portion (Kirklin and Barratt-Boyes; Type IV) and received drainage from a small left superior caval vein, which was also connected to the right superior caval vein by a bridging innominate vein. The calculated Qp:Qs was 1.4. Because of its location at the dilated distal part of the coronary sinus, the defect seemed to be suitable for a transcatheter closure using an Amplatzer® Septal Occluder. The defect was sized with a Berman catheter, which could be pulled through the defect with slight tension, and measured 6 mm. The introducer sheath passed from the right atrium through the coronary sinus defect and terminated in the left atrium. A 6 mm device was deployed with the left atrial disk within the left atrium and the right atrial disk within the coronary sinus lumen. After angiography performed with a second catheter in the left superior caval vein confirmed no residual shunt or obstruction to coronary sinus flow, the device was released. No arrhythmias were reported in the first 48 hours and follow-up echocardiography was unremarkable.
In 2007, Torres et alReference Torres, Gersony and Hellenbrand 5 used a covered stent to occlude a partially unroofed coronary sinus (Kirklin and Barratt-Boyes; Type III) in a 7-month-old infant presenting with congestive heart failure. The patient had a left superior caval vein draining to a coronary sinus with no bridging vein. Left superior caval vein angiography yielded the following measurements: left superior caval vein=7 mm, coronary sinus max diameter=1 cm, 9 mm in the area of the defect, and 8 mm at its right atrial orifice. A Rosen wire advanced from the right atrium into the left superior caval vein was used to deploy a 9 Fr, 30 mm length×12 mm diameter self-expandable Cordis-covered stent into a position where minimising the obstruction of the coronary veins but covering most of the coronary sinus atrial septal defect was required. Despite a small residual defect, the Qp:Qs ratio was reduced from 2.7 to 1.1 and resulted in an immediate resolution of tachypnea and retractions.
In 2012, Kijima et alReference Kijima, Taniguchi and Akagi 3 described their experience with two adult patients both in their 6th decade of life with exertional dyspnoea who had a coronary sinus unroofed in the terminal region (Kirklin and Barratt-Boyes; Type IV) without a left superior caval vein. Their approach was to close the orifice of the coronary sinus with an Amplatzer® Septal Occluder device, allowing sinus flow to drain to the left atrium, which from prior surgical experience was thought to be haemodynamically insignificant.Reference Attenhofer-Jost, Connolly, Danielson, Dearani, Warnes and Jamil Tajik 9 The measured Qp:Qs ratios were 1.5–1.7. Balloon stop flow technique was used to size the defects, and a device was chosen of equal size – 9 mm in both cases. The left atrial disk of the Amplatzer® Septal Occluder sat in the left atrial portion of the coronary sinus and projected out of the unroofed region of the coronary sinus into the left atrium. The disk was not large enough to occlude the roof completely and despite the sinus blood draining to the left atrium; however, both patients were reported as having “normal” systemic arterial saturations even at 24 months of follow-up. No conduction disturbances were reported.
Most recently, Santoro et al described an 8-year-old patient with similar anatomy to the patient described by Di Bernardo.Reference Di Bernardo, Fasnacht and Berger 2 , Reference Santoro, Gaio and Russo 4 Unlike Di Bernardo’s patient, the unroofed region of the coronary sinus was located more leftward within the left atrium, ∼1 cm from the coronary sinus ostium in the right atrium (Kirklin and Barratt-Boyes; Type III). The coronary sinus was enlarged and dilated secondary to drainage from a left superior caval vein. A Qp:Qs of 1.3 was reported, and device closure of the coronary sinus septal defect was performed due to the presence of right heart chamber enlargement. The defect was crossed from the right atrium with a 6-Fr multi-purpose catheter and sized with a Berman catheter. The stretched-balloon diameter was 8 mm and an equivalent-sized Amplatzer® Septal Occluder device was implanted within the coronary sinus itself. Coronary sinus angiograms in different views along with serial pressure pull-back recordings along the coronary sinus were felt to rule out any significant device obstruction of the coronary sinus. Post procedure the patient developed supraventricular tachycardia requiring pharmacotherapy. At 6-month follow-up transthoracic echocardiography detected no coronary sinus abnormalities, and ambulatory electrocardiogram monitoring detected no arrhythmias.
Discussion
The vast majority of the medical literature focuses on the more common types of atrial septal defects, particularly the secundum type for understandable reasons. There is a dearth of publications focusing on the coronary sinus atrial septal defect. The short-term surgical experience has been described in only the few aforementioned retrospective studies with small sample sizes and most are now over three decades old.Reference Ootaki, Yamaguchi, Yoshimura, Oka, Yoshida and Hasegawa 7 , Reference Attenhofer-Jost, Connolly, Danielson, Dearani, Warnes and Jamil Tajik 9 , Reference Quaegebeur, Kirklin, Pacifico and Bargeron 14 The published percutaneous device experience is limited to a few isolated case reports representing divergent anatomic subsets and technical approaches. The rarity of coronary sinus atrial septal defects has certainly been one limitation with respect to gaining significant experience with the percutaneous approach to closure, but there also appears to be a poorly substantiated reluctance in the field to pursue a percutaneous closure approach. Clearly the paucity of cases does not lend itself to traditional safety and efficacy studies; however, this does not translate into the lack of significant potential. We believe that our cases are examples that represent the potential role of the percutaneous approach in selected patients presenting with a coronary sinus atrial septal defect.
First, there remain challenges with respect to accurate diagnosis of coronary sinus atrial septal defects, and this type is more frequently overlooked due to its rarity. Non-invasive echocardiography and magnetic resonance imaging continue to have difficulty with accurate identification of coronary sinus atrial septal defects. Interestingly, thorough intracardiac echocardiography is extremely good at imaging the coronary sinus and hence, in experienced hands, will rarely miss this diagnosis when present. Unfortunately, this type of imaging cannot be performed in the outpatient setting. In addition, our second case emphasises the importance of always including intracardiac echocardiography imaging during the diagnostic catheterisation when evaluating a patient in the post-operative state who is suspected of having residual atrial level shunting by non-invasive imaging studies. Performing intracardiac echocardiography will alleviate the need for separate transoesophageal echocardiograms and duplicative sedation events.
Interventionalists have long been concerned with the effects of implanted devices on coronary sinus flow even when addressing secundum-type atrial septal defects.Reference Soo, Healy, Walsh and Wood 18 , Reference Carlson, Johnston, Jones and Grifka 19 One report describes a patient who developed left-sided chest pain radiating down the left arm and new-onset widespread T-wave inversion on an electrocardiogram following percutaneous transcatheter closure of her secundum atrial septal defect. The report states that these findings ultimately necessitated open-heart surgical removal of the device and patch closure of the defect.Reference Soo, Healy, Walsh and Wood 18 This concern is naturally amplified when considering percutaneous device closure of coronary sinus atrial septal defects. Di Bernardo et alReference Di Bernardo, Fasnacht and Berger 2 maintain that coronary sinus obstruction is unlikely if the cross-section of the configured right atrial disk of the Amplatzer® Septal Occluder is measured less than a third of the diameter of the coronary sinus. They do, however, emphasise that the more important factor appears to be the transoesophageal echocardiogram assessment of the coronary sinus flow after chosen device deployment but before release. This author recommends removal of the device if colour Doppler flow is turbulent and/or accelerated. In our view this method of determination is not reliable and may result in the misidentification of a non-obstructive device. Most of the septal occluder devices in use today allow continued “transdevice” flow for several hours after deployment, making it virtually impossible to accurately discern whether turbulent or accelerated flow is due to that which is normally associated with the device initially or due to clinically significant acute coronary sinus obstruction. Similar to previous investigators, we performed selective right and left coronary angiography with venous phase follow-through in order to help assess for the presence of clinically important coronary sinus obstruction. If a left superior caval vein is present, then selective angiography of this vessel is an excellent method of assessment as well. Torres et alReference Torres, Gersony and Hellenbrand 5 report the importance of left coronary angiography specifically to reveal the entrance of the coronary vein into the coronary sinus, which when obstructed has been associated with atrioventricular block, ventricular dysfunction, and haemodynamic decompensation. We would conclude that this would be the case only if the coronary sinus is completely occluded. In addition, it deserves mention that the coronary veins have alternative routes of drainage, which include the Thebesian veins, which can decompress the coronary veins directly into the atrial chambers or to pericardial collaterals. Cases of complete atresia of the mouth of the coronary sinus have been encountered – this author has collected angiographic teaching examples of about half a dozen such cases – and none are associated with coronary insufficiency or haemodynamic compromise. During the coronary sinus atrial septal defect procedure, careful intracardiac echocardiography appears to provide the best assessment for potential significant complete coronary sinus obstruction, and combined with angiography we feel that this can be identified whenever present.
Implanting a percutaneous device into a coronary sinus atrial septal defect is technically more demanding and challenging compared with closing a secundum atrial septal defect for several reasons. Torres et alReference Torres, Gersony and Hellenbrand 5 point out that the coronary sinus anatomy is not that of a homogenous cylindrical structure but instead takes a concave to convex longitudinal shape from the left to the right at the entrance into the atrium. Further, the morphology of the defect of the unroofed coronary sinus is not necessarily circular or oval as in most secundum atrial defects, and defining its exact morphology and the size of the rims by either transoesophageal echocardiography or balloon sizing is difficult. They also report that catheter manipulation can be challenging because of lack of room within the coronary sinus to accommodate the delivery sheath and the right disk of the device;Reference Torres, Gersony and Hellenbrand 5 however, our experience did not corroborate this finding. Beyond the often non-uniform shape of the relatively tubular coronary sinus, it should be appreciated that the terminal type (Kirklin and Barratt-Boyes; Type IV) coronary sinus atrial septal defect frequently extends beyond the formal roof of the coronary sinus into the secundum septum to varying degrees. In both of our patients we found this to be the case. This extension of the defect away from the coronary sinus helps to maintain the right disc of the septal occluder slightly away from the mouth of the coronary sinus and is an advantageous anatomic feature. Management of the coronary sinus atrial septal defect by device occlusion of the mouth of the coronary sinus – with or without the presence of a left superior caval vein – in our view, appears to be a less than desirable approach. In our patient who presented with frequent transient ischaemic attacks, one would prefer not to leave any connection between the system venous system and the left heart in order to best ensure that paradoxical neurological events do not persist. In addition, although reports of this approach insist that the patients have normal arterial saturation levels, it is unlikely that they do not experience some degree of systemic arterial desaturation at least during exercise.
In conclusion, coronary sinus atrial septal defects continue to be managed primarily by open-heart surgical repair. Although surgery is considered widely to be a “curative” therapy, the literature and our experience show that this is not the case and recurrences do occur. Contemporary transcatheter experience, along with several case reports, may suggest that percutaneous device closure can also be routinely considered in carefully selected coronary sinus atrial septal defect subtypes. As we have demonstrated, this option can result in a definitive therapeutic outcome without significant complications. Further experience with the transcatheter approach may result in the routine addition of this option; however, additional studies to establish the safety and efficacy are needed.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
Dr. Michael C. Slack is a physician proctor for St. Jude Medical.








