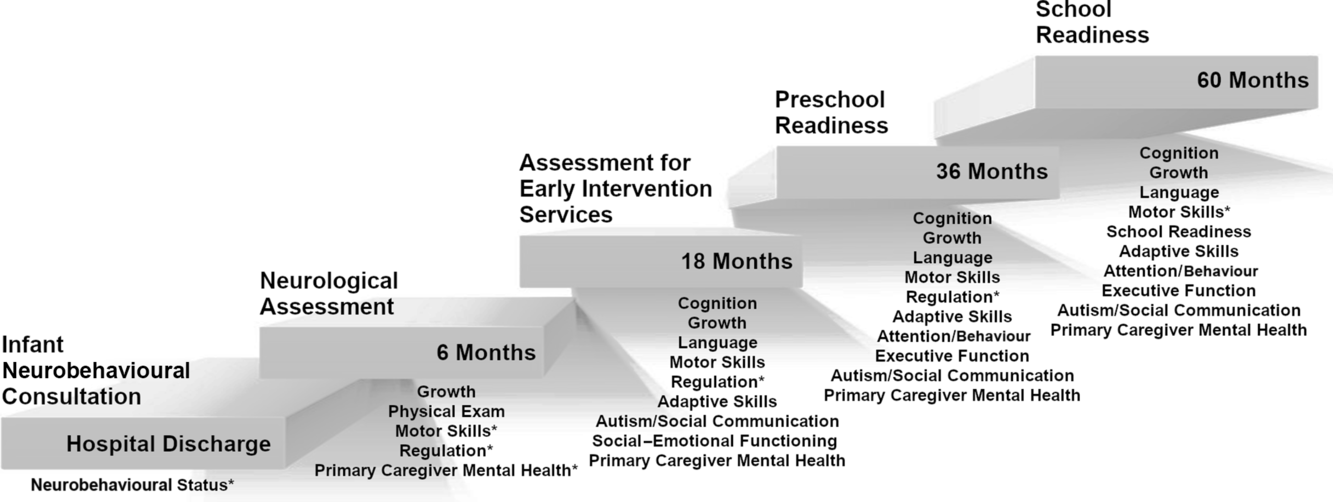Rationale
The increased survival of infants and young children born with congenital heart disease has shifted attention to reducing the neurodevelopmental risk in this vulnerable population. Reference Latal1–Reference Marelli, Miller, Marino, Jefferson and Newburger3 Young children with congenital heart disease are at-risk for a range of mild to severe disabilities and delays, often showing challenges with cognition, communication, motor skills, attention, executive functions, self-regulation, social-emotional functioning, and school readiness. These difficulties are similar in many ways to those described in children after premature birth, which have been well characterised in the literature, based on years of systematic follow-up. Reference Salt and Redshaw4–Reference Aylward7 While most countries have developed guidelines for the neurodevelopmental follow-up of preterm-born children, until recently, neurodevelopmental standards for assessment and treatment of the paediatric congenital heart disease population were unavailable. In 2012, however, the American Heart Association in conjunction with the American Academy of Pediatrics, published a Scientific Statement describing recommended practices in cardiac neurodevelopmental care, marking an important shift in the synthesis and application of the extensive literature describing the neurodevelopmental associations of congenital heart disease. Reference Marino, Lipkin and Newburger8
The scientific statement from the American Heart Association/American Academy of Pediatrics Reference Marino, Lipkin and Newburger8 highlighted the increased developmental risk for children with congenital heart disease, and the need for ongoing follow-up to maximise outcome opportunities. The statement is noteworthy for the multi-faceted follow-up strategy described, and for the specification of prescriptive tiers of neurodevelopmental care that include surveillance, screening, and evaluation. The levels of neurodevelopmental assessment and care described are cumulative in intensity, and dependent on the severity of each child’s presentation. Publication of the statement further stimulated awareness of the neurodevelopmental needs of children with congenital heart disease and promoted increased interest in the field of cardiac neurodevelopment. The American Heart Association/American Academy of Pediatrics statement was soon followed by additional publications supporting neurodevelopmental follow-up resources throughout childhood and into the adult years. Reference Rollins and Newburger9–11 Since 2012, several dozen paediatric and young adult cardiac neurodevelopmental programs have been established in medical centres across the United States, Canada, and Europe. Cardiac neurodevelopmental programs translate research findings into clinical practice in order to provide the most up-to-date services and support for children with congenital heart disease.
Soon after publication of the American Heart Association/American Academy of Pediatrics statement, a group of cardiac neurodevelopment clinicians and researchers from the US, Canada, and Europe established the Cardiac Neurodevelopmental Outcome Collaborative. The Cardiac Neurodevelopmental Outcome Collaborative 11 is a not-for-profit organisation dedicated to determining and implementing consensus neurodevelopmental practices for clinicians caring for individuals with paediatric and congenital heart disease and their families. It encompasses clinical, quality improvement, research, and public policy initiatives, and includes active committees dedicated to each goal. Committee members are drawn from a broad community of clinical, academic, and patient advocacy stakeholders.
This manuscript is the product of a Cardiac Neurodevelopmental Outcome Collaborative initiative to establish a consensus-based, standardised battery for the content and timing of neurodevelopmental assessments for children with complex congenital heart disease to promote consistent neurodevelopmental care and quality improvement, while also recognising that neurodevelopmental assessment and care must be tailored to a patient’s specific circumstances. The paper describes a model of assessment for the youngest group of congenital heart disease survivors, aged birth through 5 years. It is complemented by a second publication focusing on school-aged children with congenital heart disease. Each manuscript describes core and extended versions of age-specific assessment batteries. The content of the batteries was developed by the Cardiac Neurodevelopmental Outcome Collaborative’s Infant Working Group, a multidisciplinary group of psychologists, developmental paediatricians, neurologists, educators, cardiologists, cardiovascular surgeons, nurses, occupational and physical therapists, speech-language pathologists, social workers, and parents.
Background
The field of cardiac neurodevelopment evolved from an extensive body of research literature generated in the past 25 years, documenting early Reference Gaynor, Stopp and Wypij12–Reference Butler, Sadhwani and Stopp15 and late Reference Gaynor, Stopp and Wypij12,Reference DeMaso, Calderon and Taylor16–Reference DeMaso, Labella and Taylor20 neurodevelopmental effects of congenital heart disease. Congenital heart disease-related impairments have been identified in all developmental domains, with symptoms appearing in different forms at different ages and stages of life. Infants with congenital heart disease commonly struggle with regulatory functions, Reference Butler, Sadhwani and Stopp15,Reference Stene-Larsen, Brandlistuen and Holmstrøm21,Reference Stene-Larsen, Brandlistuen, Holmstrøm, Landolt, Eskedal and Vollrath22 and both feeding Reference Clemente, Barnes, Shinebourne and Stein23–Reference Tregay, Brown, Crowe, Bull, Knowles and Wray26 and sleeping Reference Butler, Sadhwani and Stopp15,Reference Daniels and Harrison27 skills can be disrupted. Infants and toddlers often have delayed motor, Reference Latal1,Reference Butler, Sadhwani and Stopp15,Reference Wernovsky, Lihn and Olen28–Reference Kharitonova, Marino, McCusker and Casey30 language, Reference Latal1,Reference Fourdain, St-Denis and Harvey31–Reference Goldsworthy, Franich-Ray, Kinney, Shekerdemian, Beca and Gunn34 and cognitive Reference Latal1,Reference Gaynor, Stopp and Wypij12,Reference Bellinger, Wypij and Kuban13,Reference Wernovsky and Licht29,Reference Brosig, Bear and Allen33,Reference Peyvandi, Latal, Miller and McQuillen35 milestones. In preschool and early elementary years, other challenges become more prominent, including learning delays or disability, Reference Kharitonova, Marino, McCusker and Casey30,Reference Brosig, Bear and Allen33,Reference Razzaghi, Oster and Reefhuis36–Reference Brosig, Bear and Allen39 fine and graphomotor output challenges, Reference Latal1,Reference Marino, Lipkin and Newburger8,Reference Naef, Liamlahi and Beck40–Reference Farr, Downing, Riehle-Colarusso and Abarbanell42 poor attention, Reference Wernovsky and Licht29,Reference Razzaghi, Oster and Reefhuis36,Reference Brosig, Bear and Allen39,Reference Tsao, Lee and Jeng43 and impaired social functioning. Reference Latal1,Reference Brosig, Bear and Allen33,Reference Goldsworthy, Franich-Ray, Kinney, Shekerdemian, Beca and Gunn34,Reference Brosig, Bear and Allen39,Reference Ringle and Wernovsky44 Young children with congenital heart disease are at high risk for behaviour and mood problems, Reference DeMaso, Calderon and Taylor16,Reference Oliver, Wright and Kakadekar45,Reference Cassidy, Bernstein, Bellinger, Newburger and DeMaso46 and may appear less socially and emotionally mature than typically developing peers. Reference Medoff-Cooper and Ravishankar25,Reference Costello, Gellatly, Daniel, Justo and Weir47–Reference Aguilar, Raff, Tancredi and Griffin50
Birth through five assessment battery goals
The goal of the proposed Birth through Five Assessment Battery (Fig 1) is to provide reliable assessment of early childhood development, gathered at critical developmental touchpoints, and integrated within a medical plan delivered and coordinated by the “medical home.” Reference Wernovsky, Lihn and Olen28 Using a biopsychosocial approach, this assessment battery aims to enhance our understanding of the interwoven factors that discriminate between neurotypical and atypical patterns of childhood development. The selection of assessment instruments for the battery represents consensus practice approaches, and considers early medical risk, current health status, family and caregiver considerations, and environmental contributors to developmental progress. The results generated from this assessment battery are critical for diagnosing, treating, and tracking developmental progress.
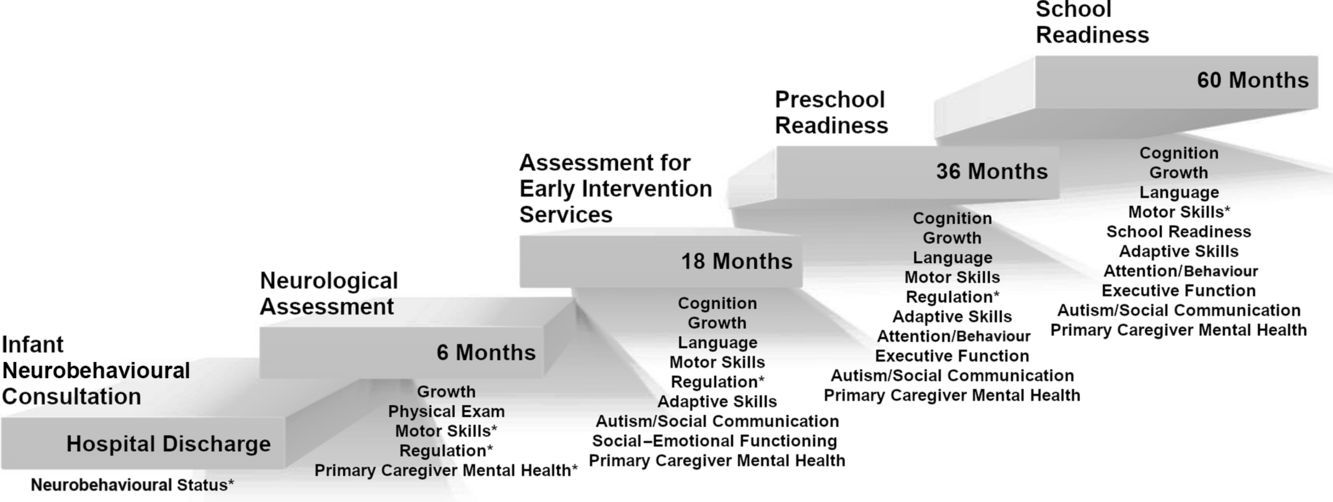
Figure 1. Congenital heart disease neurodevelopmental assessment domains for birth through 5 years of age.
*Denotes extended battery
The assessment battery as a whole addresses four themes that are dominant in choosing assessment tools appropriate for young children at identified risk for developmental, learning, and/or behavioural concerns. These include evaluation procedures that support: increasing earliest possible access to secondary prevention services through early identification of risks known to pose threats to robust outcomes, use of evidence-based practices that promote reliable test results, consideration of family and cultural preferences regarding participating in recommended early neurodevelopmental evaluation and translating and interpreting assessment findings to support multidimensional community action.
The content of the 0–5 Core Neurodevelopmental Assessment Battery is described in Tables 1 and 2. Additional data points are included in the 0–5 Extended Neurodevelopmental Assessment Battery (Table 3), which can be used to conduct more comprehensive neurodevelopmental testing. The data points from the 0–5 Core Neurodevelopmental Assessment Battery will include scores and measurements representing risk for deficits in adaptive behaviour, attention and behaviour regulation, cognition, executive function, growth, language, neurological status, motor skills, school readiness, family social support, and primary caregiver’s mental health.
Table 1. Cardiac Neurodevelopmental Outcome Collaborative 0–5 Neurodevelopmental Assessment Battery by target ages and testing times

Note: Minutes for Core and Extended Battery include caregiver questionnaires that can be completed concurrently
Table 2. Cardiac neurodevelopmental outcome collaborative 0–5 core neurodevelopmental assessment battery by test and age of administration

Note: ABAS-3 = Adaptive Behavior Assessment System: Third Edition, BASC-3 = Behavior Assessment System for Children: Third Edition, BSID = Bayley Scales of Infant & Toddler Development: Third Edition (BSID-III) or Bayley Scales of Infant & Toddler Development: Fourth Edition (BSID-4), BSID Screening Test = Bayley Scales of Infant and Toddler Development Screening Test: Third Edition (Bayley-III Screening Test) or Bayley Scales of Infant and Toddler Development Screening Test: Fourth Edition (Bayley-4 Screening Test), BRIEF-P = Behavior Rating Inventory of Executive Function-Preschool, BSRA-3 = Bracken School Readiness Assessment: Third Edition, DASS-21 = Depression, Anxiety & Stress Scale, ITSEA = Infant-Toddler Social and Emotional Assessment, MCHAT-R/F-RF = Modified Checklist for Autism in Toddlers: Revised/Follow-Up, Movement ABC-2 = Movement Assessment Battery for Children: Second Edition, SRS-2 = Social Responsiveness Scale: Second Edition, VMI-6 = Beery-Buktenica Developmental Test of Visual-Motor Integration: Sixth Edition, WPPSI-IV = Wechsler Preschool & Primary Scale of Intelligence: Fourth Edition
Table 3. Cardiac neurodevelopmental outcome collaborative 0–5 extended neurodevelopmental assessment battery by test and age of administration

Note: ABAS-3 = Adaptive Behavior Assessment System: Third Edition, BASC-3 = Behavior Assessment System for Children: Third Edition, BEARS = BEARS Sleep Screening Algorithm, BPFAS = Behavioral Pediatrics Feeding Assessment Scale, BSID = Bayley Scales of Infant & Toddler Development: Third Edition (BSID-III) or Bayley Scales of Infant & Toddler Development: Fourth Edition (BSID-4), BSID Screening Test = Bayley Scales of Infant and Toddler Development Screening Test: Third Edition (Bayley-III Screening Test) or Bayley Scales of Infant and Toddler Development Screening Test: Fourth Edition (Bayley-4 Screening Test), BRIEF-P = Behavior Rating Inventory of Executive Function-Preschool, BSRA-3 = Bracken School Readiness Assessment: Third Edition, DASS-21 = Depression, Anxiety & Stress Scale, ITSEA = Infant-Toddler Social and Emotional Assessment, MCHAT-R/F-RF = Modified Checklist for Autism in Toddlers: Revised/Follow-Up, Movement ABC-2: Movement Assessment Battery for Children: Second Edition, NBO = Newborn Behavioural Observations, PLS-5 = Preschool Language Scale: Fifth Edition, REEL-3 = Receptive-Expressive Emergent Language Test: Third Edition, SRS-2 = Social Responsiveness Scale: Second Edition, VMI-6 = Beery-Buktenica Developmental Test of Visual-Motor Integration: Sixth Edition, WPPSI-IV = Wechsler Preschool & Primary Scale of Intelligence: Fourth Edition
This assessment battery aims to promote early, accessible, and reliable identification of children with congenital heart disease who are at-risk of neurodevelopmental delay or disability. In order to be effective, the identification process needs to include expanded, ongoing caregiver and provider education about the risks of congenital heart disease to development, the preventive benefits of early identification and intervention, and direct support and advocacy for building and delivering necessary services.
Evidence-based practices that yield reliable and replicable results for the neurodevelopmental care of young congenital heart disease patients are a vital component of the recommended 0–5 Neurodevelopmental Assessment Batteries. The field of early neurodevelopmental assessment has been negatively impacted by outdated beliefs about the development of children at early and significant medical risk, which limit adherence to best practices and guidelines for neurodevelopmental assessment and treatment. The most common false belief is that a child showing developmental concerns will “catch up” with typical peers, and that it is beneficial to “wait and see” how the child develops without intervention. The concept of “catch up” was first widely used to understand the characteristic developmental delays seen in preterm infants. Reference Gong, Ji and Shan51,52 Although there are studies with mixed findings about the effectiveness of correcting for gestational age in prematurity, there is no published literature validating the concept of “catch-up” in children with medical risks who were born full-term. Advice to “wait and see” is of particular concern in light of the overwhelming volume of developmental research Reference Britto, Lye and Proulx53–Reference Estes, Munson, Rogers, Greenson, Winter and Dawson60 attesting to the value of early recognition and treatment of developmental risk. A second widely held belief among providers and educators is that drawing attention to early developmental concerns can result in stigmatisation of the child, and ultimately reduce the child’s opportunities. Reference Shonkoff61 A third obstacle is related to the life-threatening nature of congenital heart disease, the risks associated with the cardiac surgery, and the length of hospital stays. Caregivers often are so relieved following a child’s difficult hospital course that they may not recognise emerging developmental problems, or they may be encouraged by well-intended providers who report that the child “lost” time while being in the hospital, and just needs time to get back on track developmentally. The fourth obstacle to pursuing appropriate monitoring and testing is that the value of early assessment findings is often misunderstood and discounted. Together, these false beliefs limit the number of vulnerable children who receive the services they need to optimise their outcomes, especially since early studies of the first groups of very low birth weight infant survivors demonstrated efficacy for the provision of high-quality, high-frequency, early child development services. Reference Berlin, Brooks-Gunn, McCarton and McCormick62,Reference Bonnier63
The ability to reliably identify young children at risk of developmental delays or disabilities and to quickly implement treatment programs is an important public health initiative that relies on using empirically driven, best-practice strategies that rapidly link children and families with appropriate services. In addition to direct child benefits, the use of evidence-based practices has strong potential to influence public and private payers to financially support early services for children with congenital heart disease.
Family belief systems and cultural preferences exert strong influences on a family’s decision to pursue available neurodevelopmental services for young children, and to accept available resources. Assessment and intervention services are not congruent with some belief systems about child rearing roles and responsibilities, or with some beliefs about the development of children born at-risk. 64 Cultural belief systems that promote a “wait and see” approach often collide with evidence that the infant’s immature nervous system is particularly malleable, and that it responds positively to well-timed, high-quality early interventions within the family system. Reference Shonkoff61,Reference Romeo, Christodoulou and Halverson65 Practitioners can promote family acceptance of early assessment and treatment by helping families to understand the difference between early intervention and child protective services, by choosing test instruments that minimise cultural bias, by providing culturally-sensitive interpreters, by making services accessible for working caregivers, and by promoting education and knowledge to stakeholders such as paediatricians, early intervention and daycare providers, and teachers to whom caregivers may turn for advice. In addition, an important goal of neurodevelopmental assessment in young children is to engage caregivers early in the process, and to work toward a family/clinician consensus about a child’s abilities and needs. With this goal in mind, it is critical that assessors be skilled in interpreting findings from tests administered to very young children, and in explaining these results in an appropriate manner to families from a wide variety of cultural backgrounds.
Translating assessment findings into community action
Skilled neurodevelopmental evaluators must translate clinical assessment and test results into practical treatment strategies that can be implemented in the child’s natural environment. Rapid dissemination of assessment results via written report, and direct communication with the primary care provider, cardiologist, and other medical specialists, allow for collaboration in developmental support for the child. The assessor should facilitate medical referrals to specialists as appropriate (e.g., audiology, ophthalmology, neurology), as well as to developmental providers such as early intervention programs, school special education programs, or domain-specific therapists (e.g., occupational therapy, physical therapy, speech and language pathology, feeding therapy).
Early intervention services are an excellent example of a secondary prevention model designed to prevent, delay, or reduce the consequences and the severity of disease effects. In the United States, the Early Intervention Program for Infants and Toddlers with Disabilities is a federal grant program that provides funding to states to conduct comprehensive developmental services for children, birth to age 3 and their families. Early intervention content and eligibility for services are determined at the individual state level, with some states contributing additional funding to broaden the scope of their services. Early intervention in the United States is described as “low cost or no cost,” depending on the state. Funds from federal, state, and health insurance coverage combine to fund the services, with some states charging minimal fees, or insurance companies requiring co-pays for services. State-dependent eligibility criteria range from simple documentation of the presence of a developmental delay, to specific formulas requiring degree of delay (such as 1.5 standard deviations below a mean score on standardised screening tools). In some states, automatic eligibility is presumed for very young primary caregivers, and for families living with certain challenging circumstances. Funding for early intervention in Canada comes directly from the provinces, does not include federal support or legislative oversight, and may vary among provinces. Early intervention services in Canada are less standardised than they are in the United States. Canadian services are free to families as a part of community-based public health and education services. Early intervention in the European countries is defined as services for children from birth until they enter the education system, typically at the age of 6 or 7. They are free to families, and the costs are covered by public health resources and insurance coverage. Individual European countries define eligibility and content of early intervention services, and there is no central coordinating umbrella agency.
Despite extensive documentation that early intervention improves outcomes for children with early risk indicators such as preterm birth, as many as 90% of potentially eligible preterm children in the United States do not receive services, and 45% of children in the United States referred to early intervention for assessments never go on to complete the assessments. Reference Roberts, Howard, Spittle, Brown, Anderson and Doyle66 Among children meeting eligibility requirements for early intervention services, only 10–54% use available services. Reference McManus, McCormick, Acevedo-Garcia, Ganz and Hauser-Cram67 To our knowledge, recent statistics describing early intervention referral and usage patterns in countries other than the United States and Canada are not available. Cultural, economic, and educational factors are powerful determinants of whether families will use and accept early intervention. Caregivers who decline early intervention services sometimes cite the following reasons: the notion that outside involvement is not necessary for optimal development, since good care and natural development are sufficient, limited caregiver knowledge about child development and typical developmental expectations, and confusion about what early intervention is, and concern that early intervention is connected with social service agencies that may remove a child from the family. Reference Bailey, Hebbeler, Scarborough, Spiker and Mallik68–Reference Little, Kamholz, Corwin, Barrero-Castillero and Wang73 Thus, a significant public health challenge centres around how to provide accurate and accessible information to families about the significance of the developmental risks associated with complex congenital heart disease, and to improve access to early intervention for children and families.
At the age of 3 years, public schools take over assessing individual child needs in the United States, and delivering special education for those who, as a result of their disability, require special instruction in order to have appropriate access to the curriculum, and to make meaningful progress in school. In the United States, comprehensive special education services are considered federal entitlement services under the Individuals with Disabilities Education Act. 74 This Act mandates services for eligible children from birth until high school graduation, or until the 22nd birthday. The Individuals with Disabilities Education Act requires all states to find and determine special education eligibility for all children enrolled in public schools. However, there is a great deal of state-to-state latitude in determining eligibility criteria and content of Special Education services.
Once a child is found eligible for special education, an Individualised Education Plan is developed. An Individualised Education Plan is a legal document that identifies an individual child’s learning needs, outlines a detailed plan to implement remediation services, and reviews progress at least yearly so that the services provided remain relevant to the child’s changing needs. Children who are not eligible for federally-mandated special education services under the Individuals with Disabilities Education Act may qualify for Section 504 of the Rehabilitation Act 75 of 1973, a civil rights statute that guarantees children in the United States equal access to educational opportunities. Section 504 prohibits discrimination against individuals with disabilities, and provides accommodations that guarantee equal access to instruction, such as wheelchair ramps, assistive technology, extended time on tests, and movement breaks.
Clinical considerations
Access to specialised congenital heart disease assessment services
Cardiac neurodevelopmental programs are available throughout the United States, Canada, and Europe, and are typically housed within academic medical centres. A list of current Cardiac Neurodevelopmental Outcome Collaborative member institutions can be obtained by clicking on the following link: https://www.cardiacneuro.org/institutions/
Referral patterns
Children with congenital heart disease meeting American Heart Association/American Academy of Pediatrics high-risk criteria or demonstrating risk for developmental disorders or disabilities based on surveillance can be referred by medical providers, family members, early intervention programs, school systems, and therapeutic specialists to local congenital heart diseases follow-up programs or other appropriate venues for neurodevelopmental assessment.
Common concerns for young children with congenital heart disease referred to cardiac neurodevelopmental programs include delays in gross motor skills, expressive language, behaviour dysregulation, and feeding difficulties. Reference Marino, Lipkin and Newburger8,Reference Gaynor, Stopp and Wypij12,Reference Brosig, Bear and Allen33,Reference Brosig, Bear and Allen39 Young children also present to cardiac neurodevelopmental programs with dysregulated sleep patterns and with separation or medical anxiety. Reference Indramohan, Pedigo, Rostoker, Cambare, Grogan and Federman24,Reference Brosig, Bear and Allen33,Reference Brosig, Bear and Allen39,Reference Oliver, Wright and Kakadekar45,Reference Blasquez, Clouzeau and Fayon48,Reference Ykeda, Lorenzi-Filho, Lopes and Alves76,Reference Delaney, Mussatto, Slicker and Goday77 Once children reach preschool, grade retention is often considered by parents, sometimes at the recommendation of schools or providers. Grade retention recommendations are most common among children with histories of repeat or prolonged hospitalisations, and who have reduced stamina and endurance. Reference Gerstle, Beebe, Drotar, Cassedy and Marino37,Reference Mulkey, Bai and Luo38,Reference Farr, Downing, Riehle-Colarusso and Abarbanell42,Reference Oster Matthew, Stephanie, Hill Kevin, Knight Jessica and Meyer Robert78 Research is increasingly recognising that caregiver mental health challenges are prevalent in this population, including symptoms of traumatic stress and other acute stress disorders, and must be considered within the context of a child’s comprehensive neurodevelopmental assessment. Reference Woolf-King Sarah, Alexandra, Arnold Emily, Weiss Sandra and David79–Reference Golfenshtein, Hanlon, Deatrick and Medoff-Cooper85 Additionally, family members often refer a child for parental concern about developmental progress, and a question about whether catch up should be anticipated. Reference Cicchetti86
The assessment process
A comprehensive neurodevelopmental assessment in early childhood should be multidimensional and include caregiver report, direct child testing, and clinician observations. Standardised testing is an important component but must be complemented by a detailed caregiver interview of concerns emphasising both strengths and weaknesses, a review of medical, developmental, and psychosocial histories, and a summary of current special education and therapeutic intervention services. Supplemental information should, if possible, be obtained from key supports, including teachers, early intervention and childcare providers, and therapists. The assessment must include gathering behavioural data using both structured (testing) and semi-structured (observational) methods. Information about caregiver-child interactions is an important component to include in the observations in addition to the direct child testing. After the assessment, feedback and recommendations should be provided to the family, with an emphasis on giving family opportunities for discussion and questions. Feedback should be followed by a detailed written report that includes standardised test results but, most importantly, interpretation and integration of the findings into a cohesive profile of the child’s overall functioning, a clear plan of remedial action, and specific recommendations for future follow-up needs.
Addressing infant assessment controversies
Infant neurodevelopmental tests conducted by qualified clinicians are effective in identifying patterns of functioning, in assisting to design treatment programs, and in highlighting how children meet eligibility criteria for accessing early intervention services. There is little data to support using standardised infant test scores as reliable predictors of later outcomes, except among children with neurodevelopmental test scores that are well below expectations, Reference Spencer-Smith, Spittle, Lee, Doyle and Anderson87,Reference Spittle, Spencer-Smith and Eeles88 and in general, development tests administered before the age of 18 months yield variable prediction. The low predictive value of tests administered in children younger than 18 months are explained by both innate and environmental factors, including infants’ high reliance on fine and gross motor capacities for tasks that require strength, endurance, and coordination, infants’ variable ability to effectively engage and maintain cooperative attention with an unfamiliar assessor, and many infants’ lack of consistent prior exposure to formal learning experiences. After 18 months, the ability to use language to solve problems, and to demonstrate acquired knowledge, contributes to a gradual increase in the predictive accuracy of assessments, such that scores at 36 months more closely approximate scores from standard intelligence quotient measures administered later in childhood. Reference Aylward and Aylward89–Reference Yu, Hsieh and Hsu94
The Bayley Scales of Infant and Toddler Development 95,Reference Weiss, Aylward and Oakland96 has generally been considered the gold standard tool for assessing overall development in infancy and early childhood. However, follow-up of children assessed at the upper ages of the scale indicates that Bayley Scales standard scores inflate outcomes, potentially masking critical delays that would warrant early and ongoing remediation. Reference Johnson, Moore and Marlow97,Reference Goldstone, Baiocchi and Wypij98 Accurate predictive power is paramount in qualifying children for both early intervention and preschool special education services. Reference Aylward99 Thus, caution is warranted in using the Bayley Scales for describing 3-year outcomes, and instead it is recommended to use the Wechsler Preschool and Primary Scale of Intelligence, Fourth Edition 95 as the primary assessment tool to describe cognitive functioning in the early preschool years. Reference Anderson and Burnett100,Reference Luttikhuizen dos Santos, de Kieviet, Königs, van Elburg and Oosterlaan101 The fourth edition of the Bayley Scales was released and distributed in September 2019. We anticipate that the 0–5 Neurodevelopmental Assessment Battery will transition from the third to fourth edition of the Bayley Scales once this new test has been successfully used in clinical practice settings. Experience with replacing prior versions of the Bayley Scales suggests a period of 6–12 months post-distribution for implementation and transition.
The overwhelming majority of well-designed studies evaluating early test performance with later childhood test outcomes were conducted using cohorts of premature infants that followed children from infancy to young adulthood, and found both developmental effects of prematurity, as well as resilience capacity strongly related to the presence of supportive environments and available remediation. Reference Hack, Flannery, Schluchter, Cartar, Borawski and Klein102 Clinical experience with the congenital heart disease population suggests certain similarities in the developmental patterns of children born preterm and those with congenital heart disease, such as disruption of regulatory functions, gross motor delays, and risk of cognitive challenges. Reference Marino, Lipkin and Newburger8,Reference Brosig, Bear and Allen33,Reference Brosig, Bear and Allen39,Reference Wernovsky103–Reference Bhutta, Cleves, Casey, Cradock and Anand105 Thus, the basis of this battery was informed by lessons from the prematurity literature, including a decision not to combine test scores from infancy with those gathered at age 18 months and later in describing the overall performance of children with congenital heart disease.
Although it has become acceptable in the past decade to use the word “cognition” to describe the neurodevelopmental test results of very young children, the content of current neurodevelopmental tests for young children typically focusses on more than a single score or cognitive estimate. Current infant and early childhood neurodevelopmental assessments consider multiple domains of development including cognitive problem-solving abilities, language, and motor skills. Emphasis is placed on identifying patterns of strengths and weaknesses that help define treatment needs and include demonstrations of expressive and receptive language capacities, social communication skills, and the child’s functional use of discrete fine and gross motor skills.
Experienced testers are required to test young children, since this population presents with temperament and regulatory challenges that can complicate testing. In addition, the unique medical conditions of young children with congenital heart disease, particularly congestive heart failure, hypoxemia, or both, may limit the amount of testing that can be undertaken during a single testing session. Infant neurodevelopmental tests are intended to capture a child’s highest level of developmental performance, so assessors should schedule testing at the time of day when a child is maximally alert and cooperative, repeat the presentation of test items (within test guidelines) to obtain optimal performance, use breaks, flexibly select the order of test items to maintain child engagement, and use heavy reinforcement schedules to motivate persistence. Effective balancing of the simultaneous demands to maintain standardised procedures, achieve timely administration, and attend to the regulatory needs of the young child requires experienced assessors in order to obtain reliable results.
Assessor qualifications are an important consideration in generating accurate clinical diagnoses and research-quality data. Appropriately trained assessors of the primary psychological assessment instruments used in the Cardiac Neurodevelopmental Outcome Collaborative 0–5 Assessment Battery should have formal training in psychometrics, supervised experience in the use, administration, and interpretation of standardised tests, supervised experience in testing young children, and training in infant and child development. The ability to competently administer and score infant assessment tests is necessary but not sufficient given the need for interpreting and integrating the findings for well formulated feedback and report writing purposes. Cardiac Neurodevelopmental Outcome Collaborative sites have primarily used licensed psychologists and neuropsychologists for administering, scoring, and interpreting the 0–5 Neurodevelopmental Assessment Battery, although several sites use graduate level trained developmental specialists (nurse practitioners, developmental paediatricians, occupational therapists, speech therapists) to administer Bayley subscales (see Miller et al, this issue), then combining the results into a formulated report. This practice is considered acceptable by Pearson Education, Inc., the developer of the Bayley Scales, particularly when the test results are used for screening and treatment planning purposes, rather than for diagnostic specification.
Assessment accommodations for developmentally delayed and impaired children
Accurate, early, and ongoing surveillance and screening can help ensure that children deemed high risk per American Heart Association criteria are referred for more comprehensive evaluations, and appropriate resources, while lower risk children may not require the same level of detailed assessment. The high risk category for whom comprehensive neurodevelopmental assessments are recommended includes many patients who required surgical interventions within the first year of life. Important considerations in implementing effective neurodevelopmental assessments for children with congenital heart disease include the evaluator’s experience and training in the field of cardiac neurodevelopment and her/his ability to communicate test findings effectively across disciplines and settings given the multiple specialists often involved in a child’s care.
Young children with complex medical and developmental courses pose many assessment challenges that require accommodations in order to generate reliable test results. Examiners should be familiar with developmental processes, have experience testing children with physical, perceptual, and sensory differences, and strong knowledge of both typical and atypical patterns of development.
Physical testing accommodations include use of equipment designed and sized for each child’s age. For example, evaluators of toddlers and preschool-age children should use chairs that support the upper body and arms, in order to minimise the effect of low tone in the upper extremities. A sitting child’s feet should be flat on the floor, with arms comfortably resting at a 90-degree angle to the tabletop. Caregivers of children aged 3 years or younger typically remain in the room with the child during assessment, preferably seated as quiet observers, slightly behind and to the side of the child. Infants and young toddlers should sit on a caregiver’s lap at a table adjusted for the child’s reach.
Given the predominance of early visual-motor delays in children with congenital heart disease, it is especially critical to provide visual-motor accommodations that do not invalidate the tasks. Children should always wear prescribed eye glasses, for example, and test materials should sometimes be presented in enlarged format, and/or on an elevated surface. Children with motor and/or hearing impairments require a similar range of accommodations.
Common errors that compromise the results of standardised testing include substituting recommended preschool-sized writing implements with small crayons or adult-sized pencils for writing and drawing tasks, testing for gross motor skills without shoes, failing to record timing and the number of trial efforts on performance, and the need for sensitivity to fatigue to refresh endurance capacities.
Recommended timeframes for assessments
The recommended timeframes for assessment reflect sensitive periods in which developmental gains are typically observed, such as the onset of independent walking, and bursts in language development. An “age window” range in Table 1 is also included as a guide to determine test choices when a child falls outside of the target age.
Cardiac neurodevelopmental outcome collaborative birth through five assessment battery
Two versions of the Cardiac Neurodevelopmental Outcome Collaborative Birth Through Five Neurodevelopmental Assessment Battery have been developed. The Core version (Table 2) recommends an abbreviated battery that is considered the minimum necessary for adequate evaluation of the young child and is suitable for programs with limited resources. The Extended version (Table 3) includes additional diagnostic measures, as well as data from family and teacher questionnaires, addressing issues relevant to the congenital heart disease population that exert influences on outcomes not included in the Core assessment battery. Both versions include test instrument choices that represent consensus practice advice, based on a thorough review of assessment options by the Cardiac Neurodevelopmental Outcome Collaborative Infant Working Group, which is comprised of multidisciplinary cardiac neurodevelopment clinicians and researchers.
The Core battery (Table 2) focusses on a gestalt view of the child’s strengths and weaknesses, and includes assessments of cognition, language, neurological functioning, motor skills (fine and gross), social communication (including autism), attention/behaviour, adaptive functioning, and school readiness. Socio-demographic data and a brief index of caregiver mental health functioning are included in the Core battery, considering the known powerful effects these variables have on child outcomes. The Core assessment takes approximately 1–3 hours, including caregiver questionnaires that can be completed prior to the assessment or concurrently while the child testing is conducted. The assessment battery is appropriate for children aged 6 months through 5 years. The timing for completion of the assessments is age dependent.
The Extended battery (Table 3) provides for a more comprehensive neurodevelopmental assessment conducted in one or two sessions for a total of 1–4 hours, depending on the child’s age and individual needs. The Extended battery includes the variables in the Core battery and adds expanded assessment tools for language and motor development as well as evaluation of sleep and feeding regulatory capacities.
Test instrument rationale
The Cardiac Neurodevelopmental Outcome Collaborative 0–5 Neurodevelopmental Assessment Battery (Tables 4 and 5) is designed as a clinical battery, though results are also useful in research. Results from the testing are used for clinical purposes to identify patterns of strengths and weaknesses, to clarify diagnostic status, develop treatment plans, and to establish a baseline from which change can be measured over time. The specific assessment measures in the Cardiac Neurodevelopmental Outcome Collaborative 0–5 Neurodevelopmental Assessment Battery were selected on the basis of: strong normative and test construction standards, universal acceptance as gold standard measures of early childhood development, broad application across many cultures, and wide accessibility of the measures. Several of the outcome measures listed in the battery may not be useful in countries other than the United States and Canada due to lack of local normative values, unavailability of translated materials, or inapplicable culture-specific content, and other culturally appropriate measures should be considered. Due to the young child’s limited capacity to self-report, primary data sources combine direct standardised child testing with standardised parent/caregiver questionnaires.
Table 4. Cardiac Neurodevelopmental Outcome Collaborative 0–5 Core Neurodevelopmental Assessment Battery by domain, target ages, tests, and time

Note: ABAS-3 = Adaptive Behavior Assessment System: Third Edition, BASC-3 = Behavior Assessment System for Children: Third Edition, BSID = Bayley Scales of Infant & Toddler Development: Third Edition (BSID-III) or Bayley Scales of Infant & Toddler Development: Fourth Edition (BSID-4), BSID Screening Test = Bayley Scales of Infant and Toddler Development Screening Test: Third Edition (Bayley-III Screening Test) or Bayley Scales of Infant and Toddler Development Screening Test: Fourth Edition (Bayley-4 Screening Test), BRIEF-P = Behavior Rating Inventory of Executive Function-Preschool, BSRA-3 = Bracken School Readiness Assessment: Third Edition, DASS-21 = Depression, Anxiety & Stress Scale, ITSEA = Infant-Toddler Social and Emotional Assessment, MCHAT-R/F-RF = Modified Checklist for Autism in Toddlers: Revised/Follow-Up, Movement ABC-2 = Movement Assessment Battery for Children: Second Edition, SRS-2 = Social Responsiveness Scale: Second Edition, VMI-6 = Beery-Buktenica Developmental Test of Visual-Motor Integration: Sixth Edition, WPPSI-IV = Wechsler Preschool & Primary Scale of Intelligence: Fourth Edition
Table 5. Cardiac Neurodevelopmental Outcome Collaborative 0–5 Extended Neurodevelopmental Assessment Battery by domain, target ages, tests, and time
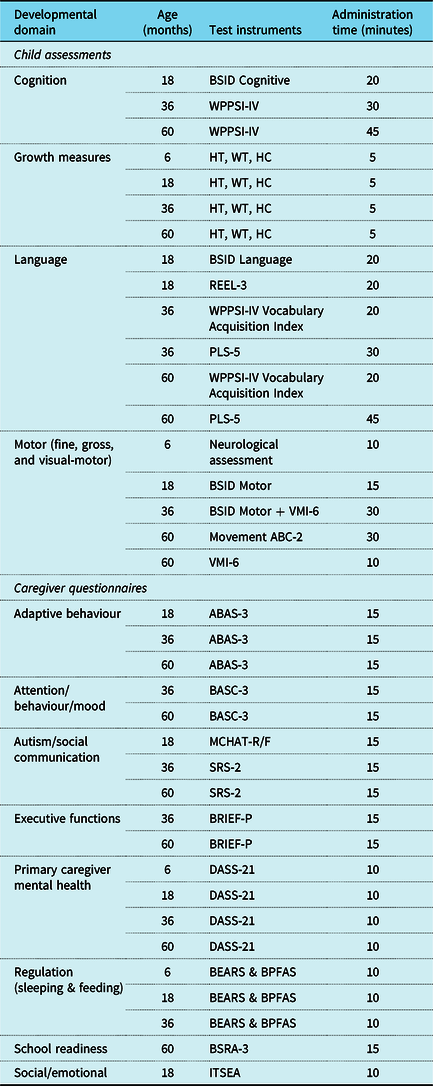
Note: ABAS-3 = Adaptive Behavior Assessment System: Third Edition, BASC-3 = Behavior Assessment System for Children: Third Edition, BEARS = BEARS Sleep Screening Algorithm, BPFAS = Behavioral Pediatrics Feeding Assessment Scale, BSID: Bayley Scales of Infant & Toddler Development: Third Edition (BSID-III) or Bayley Scales of Infant & Toddler Development: Fourth Edition (BSID-4), BSID Screening Test = Bayley Scales of Infant and Toddler Development Screening Test: Third Edition (Bayley-III Screening Test) or Bayley Scales of Infant and Toddler Development Screening Test: Fourth Edition (Bayley-4 Screening Test), BRIEF-P = Behavior Rating Inventory of Executive Function-Preschool, BSRA-3 = Bracken School Readiness Assessment: Third Edition, DASS-21 = Depression, Anxiety & Stress Scale, ITSEA = Infant-Toddler Social and Emotional Assessment, MCHAT-R/F-RF = Modified Checklist for Autism in Toddlers: Revised/Follow-Up, Movement ABC-2 = Movement Assessment Battery for Children: Second Edition, NBO = Newborn Behavioural Observations, PLS-5 = Preschool Language Scale: Fifth Edition, REEL-3 = Receptive-Expressive Emergent Language Test: Third Edition, SRS-2 = Social Responsiveness Scale: Second Edition, VMI-6 = Beery-Buktenica Developmental Test of Visual-Motor Integration: Sixth Edition, WPPSI-IV = Wechsler Preschool & Primary Scale of Intelligence: Fourth Edition
A brief description of the focus of each component of the 0–5 Neurodevelopmental Assessment Battery includes the following.
Pre-discharge neurobehavioural consultation
If possible, before initial hospital discharge, infants with diagnoses of congenital heart disease should receive a neurobehavioural consultation, including a measure such as the Neonatal Behavioral Observation Scale Reference Nugent, Keefer, Minear, Johnson and Blanchard106 or Bayley Scales of Infant and Toddler Development Screening Test, parent interview, and family support that includes anticipatory guidance about outpatient treatment resources. The infant developmental screening component should include a review of the infant’s autonomic, motor, regulatory, and attentional systems, along with information about the infant’s feeding and sleeping patterns. The parent interview should include assessment of family stressors, a review of available support systems, and parental mental and physical status that may affect caregiving and financial resources. Specific recommendations to support areas of developmental weaknesses should be shared with the family and the medical team, including inpatient and outpatient providers. Community resource information and educational advocacy guidance should be provided. Infants determined to be at high-risk for developmental delay following the American Heart Association/American Academic of Pediatrics criteria Reference Marino, Lipkin and Newburger8 should be referred to early intervention. Newborn and infant neurobehavioural consultations are typically conducted by a psychologist, developmental-behavioural paediatrician, neonatal or behavioural neurologist, paediatric nurse practitioner, or an occupational therapist, all of whom have specialised training and experience in evaluating very young, medically at-risk children.
Infant neurological assessment
The first-year assessment has a target age of 6 months with a window of 6–12 months. The evaluation includes detailed medical, social, and family history, a neurological examination, and recommendations for outpatient therapy. Key components of the history include a review of the child’s developmental progress, a discussion of regulation including sleeping and feeding routines, and identification of family stress. The medical examiner should identify and manage medical factors that may influence neurodevelopment. Common risks to neurodevelopment identified during the neurological examination include ongoing cardiac care needs, consequences of prolonged hospital stays, the need for extracorporeal membrane oxygenation treatment, specific brain injuries such as stroke or white matter injury, seizures, and/or underlying genetic disorders. All children should have hearing testing completed in accordance with the American Heart Association/American Academy of Pediatrics statement. Reference Marino, Lipkin and Newburger8 The physical examination includes a standard neurological examination with particular attention to tone, symmetry, and gross motor skills. Note should be made of growth trajectories, especially head circumference, and any dysmorphology. Additional testing should be guided by the patient’s history and physical examination and may include genetic testing, further hearing or vision testing, a brain magnetic resonance imaging, or an electroencephalogram when clinically appropriate. Abnormalities on the neurological examination may support further testing, give insight into causes of developmental delays, and in some cases provide reassurance and empowerment to families. Depending upon the institution, the examination is most often conducted by a neonatal or behavioural neurologist, developmental–behavioural paediatrician, or paediatric nurse practitioner. This provider is part of the overall neurodevelopmental team, providing the medical context for future neurodevelopmental assessors as well as for family and community caregivers in close collaboration with the medical home provider and cardiologist.
The Bayley Scales and Adaptive Behavior Assessment System are not included in the Cardiac Neurodevelopmental Outcome Collaborative Core Assessment Battery for the first-year evaluation (Table 2), due to the reduced predictive value of both instruments under the age of 18 months. The Bayley Scales and Adaptive Behavior Assessment System are used in some cardiac neurodevelopment programs in the first year of life as supplementary measures as a descriptor of current functioning rather than a predictive instrument and to guide initial therapeutic recommendations. Screening of primary caregiver mental health status with the Depression Anxiety Stress Scale is recommended for both the Core and Extended batteries.
18-month neurodevelopmental assessment
The second-year assessment has a target age of 18 months, with a window of 13–29 months. The Core Neurodevelopmental Assessment Battery includes direct child testing and caregiver questionnaires to obtain baseline assessments of cognitive, language, and motor skills, adaptive behaviour, social–emotional functioning, growth, and primary caregiver mental health. Direct child testing includes the complete Bayley Scales (cognitive, language, and motor) and growth trajectories. Caregiver questionnaires include adaptive behaviour (Adaptive Behavior Assessment System), autism/social communication screening (Modified Checklist for Autism in Toddlers), social–emotional evaluation (Infant Toddler Social Emotional Assessment), and primary caregiver mental health screening (Depression Anxiety Stress Scale). The Extended Assessment Battery includes the addition of a neurological exam, expressive and receptive language (Receptive-Expressive Emergent Language Test), and regulatory sleeping and feeding caregiver questionnaires (BEARS Questionnaire and Behavioral Pediatric Feeding Assessment for Children).
36-month neurodevelopmental assessment
The third year evaluation has a target age of 36 months, with a window of 30–47 months. An important purpose of the 36-month assessment is to determine if preschool special education services are warranted, to assess overall progress, and to characterise early intellectual functioning using standardised tools. At this visit, the primary psychological assessment instrument shifts from the Bayley Scales as a measure of overall functioning to administration of an initial standardised intelligence quotient measure (Wechsler Preschool and Primary Scale of Intelligence, plus Vocabulary Acquisition Index). Direct child testing also includes the Beery Test of Visual Motor Integration. Caregiver questionnaires assess adaptive behaviour (Adaptive Behavior Assessment Scales), attention and behaviour regulation (Behavior Assessment System for Children), autism/social communication (Social Responsiveness Scale), executive function (Behavior Rating Inventory of Executive Function), and primary caregiver mental health concerns (Depression Anxiety Stress Scale). The Extended Assessment Battery includes additional direct child testing of language (Preschool Language Scale), and regulatory sleeping and feeding caregiver questionnaires (BEARS Questionnaire and Behavioral Pediatric Feeding Assessment for Children). If the assessor is unable to establish a basal level on the Wechsler Preschool and Primary Scale of Intelligence, then it is recommended to drop back to using the Bayley Scales in place of the Wechsler Preschool and Primary Scale of Intelligence as the primary outcome measure. The Bayley Scales provides more flexibility in administration procedures and offers a wider range of developmental test items suitable to accommodate children who may have delays in development.
60-month neurodevelopmental assessment
The fifth-year evaluation has a target age of 60 months, with a window of 48–71 months. Assessment at this age helps determine a child’s school preparedness. The Core Assessment Battery includes an updated intelligence quotient assessment (Wechsler Preschool and Primary Scale of Intelligence plus Vocabulary Acquisition Index), a kindergarten readiness screening (Bracken School Readiness Assessment, Receptive), and a visual-motor skills evaluation (Beery-Buktenica Visual-Motor Integration). Caregiver questionnaires include adaptive behaviour (Adaptive Behavior Assessment Scale), attention and behaviour regulation (Behavior Assessment System for Children), autism/social communication (Social Responsiveness Scale), executive functions (Behavioral Rating Inventory of Executive Function), and primary caregiver mental health (Depression Anxiety Stress Scale). The Extended Assessment Battery includes additional direct child testing of language (Preschool Language Scale), motor skills (Movement Assessment Battery for Children), and regulatory sleeping and feeding caregiver questionnaires (BEARS Questionnaire and Behavioral Pediatric Feeding Assessment for Children). If the child is significantly delayed and unable to establish a basal level, the test assessor discontinues the Wechsler Preschool and Primary Scale of Intelligence and switches to the Bayley Scales, obtaining raw scores and age equivalents in place of standard scores.
Refer to Table 4 for a visual summary of the Core Neurodevelopmental Assessment Battery details across ages and Table 5 for the Extended Neurodevelopmental Assessment Battery details.
Interim, additional, assessments are recommended for clinical purposes on an as-needed basis and are particularly helpful in determining whether developmental rates of progress are meaningful, or if current therapies and special education services need to be intensified or otherwise altered. Standardised tests are periodically updated. As new versions of the tests listed in the Core and Extended batteries are published, older test versions should be replaced with the newer versions to assure maximal clinical utility.
Summary
As children with congenital heart disease are increasingly surviving into adulthood, caregivers and families must respond to the neurodevelopmental vulnerabilities observed in this population. Infants and young children with congenital heart disease commonly face neurodevelopmental challenges such as motor and language delays, attention and behavioural problems, sleep and feeding difficulties, and anxiety. Reference Wernovsky103 Given the known risks associated with congenital heart disease and the demonstrated benefit of early intervention in other populations, regular monitoring and periodic neurodevelopmental assessment are critical throughout childhood in order to optimise the neurodevelopmental outcomes and the quality of life of patients with congenital heart disease. A reassuring examination during infancy is not always predictive of typical long-term development, as children are faced with increasingly complex tasks and activities as they get older. As a result, following scheduled follow-up protocols is important for all children with congenital heart disease, regardless of their presentation early in life.
This article builds on the 2012 American Heart Association/American Academy of Pediatrics Scientific Statement Reference Marino, Lipkin and Newburger8 by proposing a systematic and standardised approach to assessing the neurodevelopmental needs of children with complex congenital heart disease aged birth through 5 years. The proposed strategy describes components for both a shorter Core and a longer Extended evaluation, which are intended to provide a state-of-the-art assessment of the neurodevelopment of children at risk. The use of standardised neurodevelopmental assessment tools yielding normative values are embedded in the battery for quality improvement and clinical research purposes.
Standard application of the Cardiac Neurodevelopmental Outcome Collaborative 0–5 Neurodevelopmental Assessment Battery across cardiac neurodevelopment sites holds enormous promise for furthering both clinical care and research within the congenital heart disease population. Clinically, wide adoption of recommended practice assessment will increase the percentage of children with congenital heart disease who are accurately identified with neurodevelopmental delays and disabilities, and who receive appropriate early intervention services that are known to optimise outcomes. If applied as designed, the Cardiac Neurodevelopmental Outcome Collaborative 0–5 Neurodevelopmental Assessment Battery will help the field of cardiac neurodevelopment support each child to reach his or her full potential, and to bring the field’s understanding of cardiac neurodevelopment and optimal patient care to an unprecedented level of depth and subtlety.
Acknowledgements
This work represents the team efforts of the Infant Working Group, a multidisciplinary subcommittee within the Cardiac Neurodevelopmental Outcome Collaborative, a not-for-profit organization dedicated to advancing clinical, quality improvement, and research related to the development of children with pediatric and congenital heart disease. Each author assisted with the concept and design for the paper, the writing and editing of the narrative, and the final review.
Financial support
The authors received no financial support for the research, authorship, or publication of this article.
Conflicts of interest
None.