In the 1970s, early palliation for newborns with hypoplastic left heart syndrome involved stabilising the transitional circulation. In 1983, Norwood et alReference Norwood, Lang and Hansen1 reported the first patient successfully staged through to a Fontan operation. Subsequent outcomes for patients with hypoplastic left heart syndrome have improved from certain death before palliation to over 95% early survival at some centres.Reference Tweddell, Ghanayem and Mussatto2–Reference Furck, Uebing and Hansen6 Attributing to survival are concepts such as
• an understanding of balanced circulation,
• surgical palliation that maximises coronary perfusion and avoids obstruction of the aortic arch, and
• the early recognition of subtle haemodynamic instabilities.
The current goals have shifted from survival alone to avoidance of circulatory collapse, maximising somatic and pulmonary arterial growth, and optimising neurodevelopmental outcomes.
Pre-operative management
The incidence of foetal diagnosis varies by centre from 60% to 85%.Reference Atz, Travison and Williams7 The impact of foetal diagnosis on the early outcomes of newborns with hypoplastic left heart syndrome has been evaluated with controversial results.Reference Srinivasan, Sachdeva and Morrow4, Reference Rychik, Szwast and Natarajan8 Despite the overall lack of statistically significant improvement in outcomes following foetal diagnosis, the general impression is that, in the current era, it is less common for a neonate to present in shock with previously undiagnosed hypoplastic left heart syndrome.
In a stable newborn with hypoplastic left heart syndrome, a trend exists towards minimising intervention before surgery. For example, pre-operative intubation for a stable newborn with hypoplastic left heart syndrome is diminishing in frequency. Earlier concerns of apnoea may be negated by a gradual decrease in the dose of prostaglandins. Some centres have found pre-operative mechanical ventilation to be associated with worse outcomes.Reference Azakie, Merklinger and McCrindle9
Pre-operative efforts to balance the pulmonary and systemic circulations have also fallen from favour. Debates in the 1990s over the administration of inspired gases to decrease the flow of blood in the pulmonary circulation were addressed in 2001 when a comparative study demonstrated improved cerebral oxygenation with inspired carbon dioxide, which was not seen with inspired nitrogen.Reference Tabbutt, Ramamoorthy and Montenegro10 However, a majority of centresReference Wernovsky, Ghanayem and Ohye11, Reference Johnson, Mussatto, Uhing, Zimmerman, Tweddell and Ghanayem12 steer away from inspired gases and or controlled hypoventilation and accept the quiet tachypnoea associated with pulmonary over-circulation in the pre-operative period in the absence of compromise of end organ(s).
Initiation of pre-operative enteral feeds varies considerably between centres.Reference Wernovsky, Ghanayem and Ohye11, Reference Johnson, Mussatto, Uhing, Zimmerman, Tweddell and Ghanayem12 Older reports found an increased incidence of necrotising enterocolitis in infants with left-sided obstruction of outflow such as hypoplastic left heart syndrome.Reference McElhinney, Hedrick and Bush13, Reference Jeffries, Wells, Starnes, Wetzel and Moromisato14 However, this increased incidence of necrotising enterocolitis was in a period when a higher dose of prostaglandins was used and pre-operative intervention to control the ratio of pulmonary to systemic flow of blood was standard of care. In the absence of data, theoretical reasons for initiating pre-operative feeds include stimulation of the bowel and earlier time to full post-operative enteral feeds. Some institutions use levels of lactate or monitoring with Near Infrared Spectroscopy to determine whether systemic output supports adequate perfusion of the bowel for enteral feeds.
Despite improved and less aggressive pre-operative management, the optimal timing of surgery remains uncertain. Early palliative surgery may be complicated by post-operative hypoxaemia as the pulmonary vascular resistance is still elevated, whereas later palliative surgery puts the neonate at risk for the morbidities associated with intensive care.
Surgical reconstruction
Augmentation of the aortic arch
Creation of an unobstructed pathway from the right ventricle to the systemic circulation is a primary goal of neonatal surgery for hypoplastic left heart syndrome. Surgical strategy is determined partly by the underlying anatomy. Several techniques have been described that provide consistent results.Reference Norwood, Lang and Hansen1, Reference Jonas, Lang, Hansen, Hickey and Castaneda15–Reference Griselli, McGuirk and Stumper20 Residual or recurrent obstruction of the aortic arch occurs with a reported incidence of 11–37% and can be associated with reduced ventricular function and poor outcome.Reference Norwood, Kirklin and Sanders21 Efforts to reduce obstruction of the aortic arch have primarily focused on
• augmentation with autologous tissue versus augmentation with a non-autologous patch,
• the type of material used for the patch, and
• excision of the coarctation.
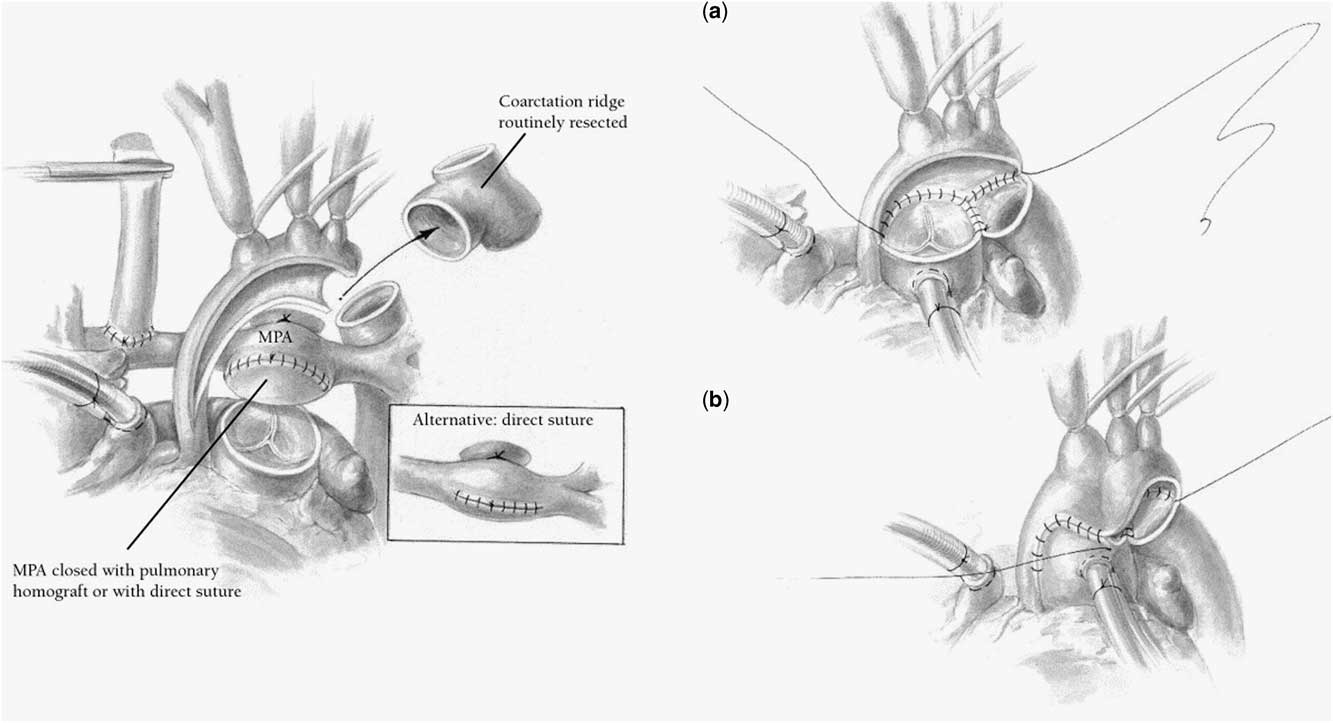
Norwood described the reconstruction of the aortic arch for hypoplastic left heart syndrome as a direct anastomosis of the transected end of the proximal pulmonary artery to the ascending aorta and aortic arch.Reference Norwood, Lang and Hansen1, Reference Norwood, Kirklin and Sanders21, Reference Norwood, Lang, Castaneda and Campbell22 Subsequently, Jonas et alReference Jonas, Lang, Hansen, Hickey and Castaneda15 described the creation of a side-to-side Damus–Kaye–Stansel connection between the transected end of the pulmonary artery and the adjacent portion of the incised ascending aorta, and the use of a patch of homograft to augment the aortic arch from the Damus–Kaye–Stansel reconstruction – pulmonary annulus – along the proximal aorta to the descending aorta well beyond the insertion of the arterial duct (Fig 1). This technique provides flexibility for variations in the anatomy of the aortic arch, decreasing the likelihood of distortion of the proximal aorta.

Figure 1 Reconstruction of the aortic arch for hypoplastic left heart syndrome utilising a side-to-side Damus–Kaye–Stansel and augmentation of the aortic arch with a patch of homograft extended from the Damus–Kaye–Stansel anastomosis to beyond the insertion of the arterial duct into the descending aorta. Source: Meyer and Spray.Reference Meyer and Spray17
Total autologous reconstruction of the aortic arch was subsequently revisited because of concerns of degeneration of the patch of homograft, calcification, and limited potential for growth.Reference Brawn16, Reference Bu'Lock, Stumper and Jagtap23–Reference Fraser and Mee24 The segment of coarctation is resected, the back wall of the descending thoracic aorta and distal aortic arch are directly anastomosed, and the underside of the aortic arch is augmented with the transected end of the pulmonary artery (Fig 2). This strategy may be most amenable to patients with a small segment of coarctation and a larger aorta. Although a recent report suggests that the technique can be widely applied and provide for excellent results,Reference Sano, Ishino, Kawada and Honjo18 the reported anatomical risk factors for this technique include

Figure 2 Total autologous reconstruction of the artic aortic arch for hypoplastic left heart syndrome, described by Bu'Lock et al.Reference Bu'Lock, Stumper and Jagtap23 Source: Brawn.Reference Brawn16
• a long and diminutive ascending aorta, with a diameter of less than 2 millimetres
• ductal tissue in the aortic arch with coarctation between the left carotid artery and subclavian artery
• a long arterial duct with a short descending thoracic aorta and
• an aberrant right subclavian artery.Reference Griselli, McGuirk and Stumper20, Reference Ishino, Stumper and De Giovanni25
Other authors have indicated that this technique of total autologous reconstruction of the aortic arch may be associated with stenosis of the left pulmonary artery and left bronchus.Reference Griselli, McGuirk and Stumper20, Reference Baker, Wells, Derby, Rizi and Starnes26
Brawn et al recently described their experience with a technique in which the aortic arch is incised on its inner aspect from the ascending aorta to distal to the arterial duct, the aortic isthmus is left in situ for those with limited or small coarctation, and the entire underside of the aortic arch is augmented with a teardrop-shaped patch of homograft.Reference Griselli, McGuirk and Stumper20 The transected end of the pulmonary artery is then anastomosed to a longitudinal incision in the patch of allograft on the underside of the reconstructed aortic arch (Fig 3). This reconstruction is purported to be technically easier and more consistently applied to all subsets of anatomy of the aortic arch. Although the incidence of re-coarctation is similar to other strategies, this technique may result in a lower rate of stenoses of the left pulmonary artery and left bronchus.Reference Griselli, McGuirk and Stumper20

Figure 3 Reconstruction of the aortic arch with augmentation with a patch for hypoplastic left heart syndrome, described by Griselli et al.Reference Griselli, McGuirk and Stumper20Source: Brawn.Reference Brawn16
The type of material used for the patch for reconstruction of the aortic arch varies. Reconstruction of the aortic arch utilising a patch created from pulmonary homograft has been recently shown to be associated with obstruction of the aortic arch. Homograft use is associated with the development of panel reactive antibodies,Reference Bautista-Hernandez, Marx and Gauvreau27–Reference Laing, Ross and Meyer30 increasing the risk for cardiac transplantation. The use of bovine pericardium or autologous pericardium for the patch for augmentation of the aortic arch appears to be durable and has been shown to result in a lower incidence of re-coarctation at early follow-up.Reference Bautista-Hernandez, Marx and Gauvreau27, Reference Morell and Wearden31
Resection of the coarctation remains controversial. Griselli et alReference Griselli, McGuirk and Stumper20 reported that obstruction of the neoaortic arch is not reduced by excision of the coarctation. Others have indicated that aggressive resection of the coarctation and associated ductal tissue with an interdigitating reconstruction of the back wall of the aorta significantly reduces the incidence of re-coarctation.Reference Bautista-Hernandez, Marx and Gauvreau27, Reference Burkhart, Ashburn and Konstantinov32 Bautista-Hernandez et alReference Bautista-Hernandez, Marx and Gauvreau27 suggest the presence of a coarctation on pre-operative echocardiogram is a risk factor for re-coarctation.
Selection of the type of shunt
The classic approach to provide pulmonary blood flow in the Norwood (Stage 1) operation is the use of a modified Blalock–Taussig shunt. The modified Blalock–Taussig shunt provides antegrade flow of blood in the pulmonary circulation during both systole and diastole. Theoretically, as the flow of coronary blood occurs primarily in diastole, “coronary steal” could occur leading to myocardial ischaemia, cardiac dysfunction, and potentially death. Some of the advantages of using a modified Blalock–Taussig shunt include
• familiarity of the surgeon with this type of shunt,
• facilitation of providing regional cerebral perfusion during reconstruction of the aortic arch, and
• setting up the second stage operation.
The modified Blalock–Taussig shunt is performed by placement of a tube of polytetrafluoroethylene, usually with a diameter of 3, 3.5, or 4 millimetres, from a branch of the aorta, usually the innominate artery, to the right pulmonary artery.
The modification of the Norwood (Stage 1) operation that includes a shunt from the right ventricle to the pulmonary artery shunt was reintroduced more recently as an alternative approach to provide flow of blood in the pulmonary circulation in the hopes of reducing the rate of mortality associated with the Norwood (Stage 1) operation.Reference Sano, Ishino, Kawada and Honjo18 The shunt from the right ventricle to the pulmonary artery results in higher diastolic systemic arterial pressure compared with the modified Blalock–Taussig shunt. The shunt from the right ventricle to the pulmonary artery requires the creation of a ventriculotomy in the systemic ventricle for the proximal anastomosis of a tube of polytetrafluoroethylene, usually with a diameter of 5–6 millimetres, to the ventricle. The distal end of the graft is then sewn to the bifurcation of the pulmonary artery. The shunt from the right ventricle to the pulmonary artery often leads to impaired growth of the pulmonary artery and the need to perform the second stage operation at an earlier age, compared with the modified Blalock–Taussig shunt.Reference Ohye, Sleeper and Mahony33, Reference Tabbutt, Dominguez and Ravishankar34 Potential disadvantages of the shunt from the right ventricle to the pulmonary artery include right ventricular dysfunction and arrhythmia related to the ventriculotomy. If regional cerebral perfusion is to be performed in conjunction with a shunt from the right ventricle to the pulmonary artery, one of the following techniques is required:
• placement of a separate graft to the innominate artery, similar to placement of the proximal end of the modified Blalock–Taussig shunt, as a route to provide regional cerebral perfusion,
• cannulation of the innominate artery with the arterial cannula placed into the aorta and advanced into the innominate artery, or
• direct cannulation of the innominate artery with the arterial cannula.
For operations performed without regional cerebral perfusion, these technical modifications are not an issue.
A number of case series comparing early outcomes of the modified Blalock–Taussig shunt and the shunt from the right ventricle to the pulmonary artery have been reported with conflicting results.Reference Tabbutt, Dominguez and Ravishankar34–Reference Pizarro, Malec and Maher38 This controversy led to a randomised clinical trial at 15 North American centres with 555 patients enrolled, conducted by the Pediatric Heart Network of the National Institutes of Health of the United States of America from May, 2005 to July, 2008.Reference Ohye, Sleeper and Mahony33 This trial is named the Single Ventricle Reconstruction trial. Patients were randomised to receive a modified Blalock–Taussig shunt or a shunt from the right ventricle to the pulmonary artery. At 12 months, transplantation-free survival was significantly higher in subjects randomised to the shunt from the right ventricle to the pulmonary artery compared with the modified Blalock–Taussig shunt. However, the subjects with a shunt from the right ventricle to the pulmonary artery had a significantly higher rate of unintended interventions and complications. At 32 plus or minus 11 months follow-up, no significant difference in transplantation-free survival was evident between the two types of shunts.
Despite the results of the Single Ventricle Reconstruction trial, no consensus exists regarding the optimal type of shunt for the Norwood (Stage 1) operation. One potential limitation to the Single Ventricle Reconstruction trial is the likelihood of a “learning curve effect”. Many surgeons and centres performed one type of shunt preferentially before the study. This fact could potentially result in a disadvantage in the trial for the less frequently used type of shunt. Not reported in the Single Ventricle Reconstruction trial was the historical preference for the type of shunt of the surgeon and centre before the initiation of the trial. Some advocates of the shunt from the right ventricle to the pulmonary artery have speculated that despite the learning curve for the newer shunt from the right ventricle to the pulmonary artery, the outcomes remained favourable for these subjects compared with those with the modified Blalock–Taussig shunt.Reference Tabbutt, Goldberg and Ohye39 However, the same can be applied to the modified Blalock–Taussig shunt for subjects at centres that preferred the shunt from the right ventricle to the pulmonary artery before the trial.
The optimal type of shunt may be specific to a particular surgeon and/or centre, or to specific innate characteristics of the patient, such as anatomy or weight at birth. Preference of the type of shunt can be a very personal decision based on the perception of a surgeon of what is best for their patients. A change in the type of shunt is not simple and may affect other important steps in the Norwood (Stage 1) operation. Recent developments may improve outcomes after both the modified Blalock–Taussig shunt and shunt from the right ventricle to the pulmonary artery, such as the introduction of the heparin-bonded polytetrafluoroethylene graft, which may decrease the incidence of thrombosis of the shunt. Further follow-up from the Single Ventricle Reconstruction trial is pending and may shed additional light on the topic.
Strategies of perfusion
Significant debate exists over the best strategy of perfusion to provide adequate cerebral protection during reconstruction of the aortic arch. Many observational studies and clinical trials directed at neuroprotective strategies have focused on the intraoperative management for congenital cardiac disease. Cardiopulmonary bypass exposes the blood to the foreign surfaces of the circuit initiating a systemic inflammatory response. When continuous regional cerebral perfusion and cardiopulmonary bypass are utilised, perfusion to the body and brain are maintained; however, the duration of exposure of the blood to the circuit used for cardiopulmonary bypass is increased compared with the strategy of deep hypothermic circulatory arrest with cardiopulmonary bypass. Deep hypothermic circulatory arrest has many advantages including
• ease of cannulation;
• an uncluttered and bloodless surgical field that may facilitate completion of the best physiologic repair; and
• shorter duration of exposure of blood to the circuit used for cardiopulmonary bypass, but at the cost of a period of global cerebral ischaemia.
There has been lingering concern that deep hypothermic circulatory arrest increases the risk of injury to the brain and adverse long-term neurodevelopmental outcomes.
Clinical trials assessing and comparing intraoperative strategies of perfusion have provided conflicting data. The most comprehensive data and longest-term follow-up come from the Boston Circulatory Arrest Study: a randomised trial of deep hypothermic circulatory arrest and continuous cardiopulmonary bypass in children undergoing the arterial switch operation – plus or minus closure of an associated ventricular septal defect.Reference Newburger, Jonas and Wernovsky40 However, patients randomised to continuous cardiopulmonary bypass did undergo a brief period of deep hypothermic circulatory arrest. Investigators found that longer periods of deep hypothermic circulatory arrest were associated with more frequent abnormalities in neurological markers in the immediate post-operative period and at 1-year follow-up. By the 8-year evaluation, the impact of use of deep hypothermic circulatory arrest was only significant for durations greater than 40 minutes.Reference Bellinger, Wypij and duDuplessis41 Performance of the entire cohort for many neurodevelopmental domains was worse than norms of the population. Assignment of treatment resulted in different neurological morbidities rather than reducing their frequency or severity. Patient-specific factors, such as socio-economic status and presence of a ventricular septal defect, explained more of the variance in neurological outcome than did treatment group. It was concluded that shorter durations of deep hypothermic circulatory arrest – less than 40–45 minutes – did not negatively influence long-term neurological outcomes compared with continuous cardiopulmonary bypass.
In a prospective observational study at The Children's Hospital of Philadelphia of survivors of cardiac surgery during infancy, deep hypothermic circulatory arrest was evaluated as a predictor of adverse neurodevelopmental outcomes at 4 years.Reference Fuller, Rajagopaian and Jarvik42 Deep hypothermic circulatory arrest was utilised selectively at the discretion of the surgeon. Children in whom deep hypothermic circulatory arrest was utilised
• were more likely to have a functionally univentricular heart,
• were younger and smaller at the time of the initial surgery,
• were more likely to undergo additional surgery with cardiopulmonary bypass,
• were more likely to have been mechanically ventilated before surgery, and
• had longer post-operative length of stay.
Despite the increased prevalence of these risk factors in the patients treated with deep hypothermic circulatory arrest, no difference was observed for unadjusted outcomes between the groups for any neurodevelopmental domain tested. Even after adjustment for covariates using multivariable linear regression, use of deep hypothermic circulatory arrest was not predictive of a worse outcome. In addition, the duration of deep hypothermic circulatory arrest was not found to be a risk factor for worse neurodevelopmental outcomes at testing at 1 year in infants undergoing staged palliation for hypoplastic left heart syndrome.Reference Tabbutt, Nord and Jarvik43 These findings support the hypothesis that use of deep hypothermic circulatory arrest during cardiac surgery in infancy is not associated with worse neurodevelopmental outcomes.
Clinicians at the University of Michigan have conducted the only randomised clinical trial comparing the strategies of regional cerebral perfusion versus deep hypothermic circulatory arrest on safety and neurodevelopmental outcomes in patients with hypoplastic left heart syndrome and variants undergoing the Norwood (Stage 1) operation (2001–2005, n = 77).Reference Goldberg, Bove and Devaney44 In this study, all patients were cooled to 18 degrees Celsius. For subjects receiving regional cerebral perfusion, cerebral flow was delivered through a cannula in a tube of polytetrafluoroethylene connected to the innominate artery, initiated at 5 millilitres per kilogram per minute, and increased to 20 millilitres per kilogram per minute. The average duration of deep hypothermic circulatory arrest was 5.7 minutes for the cohort managed with regional cerebral perfusion and 41 minutes for the cohort managed with deep hypothermic circulatory arrest. No differences were found between groups in terms of survival to discharge, survival to second stage operation, or survival to 1 year of age. Neurodevelopmental testing using the Bayley Scales of Infant Development II was performed before the second stage operation and again at 1 year of age. No significant differences were found between groups in either the psychomotor development index or the motor development index. The authors recommended that a consensus be reached on the approach to regional cerebral perfusion, including the rate of flow, position of the cannula, and temperature. Once accomplished, a multi-centric clinical trial would likely to be needed to determine whether a particular strategy of perfusion provides improvement in neurodevelopmental outcomes without increasing the rate of early morbidities and mortality for children undergoing the Norwood (Stage 1) operation.
To determine nationwide patterns of practice, 140 surgeons were surveyed as to their approach to strategies of perfusion, and deep hypothermic circulatory arrest versus regional cerebral perfusion.Reference Ohye, Goldberg and Donohue45 Routine or exclusive use of deep hypothermic circulatory arrest was reported by 20% of respondents compared with 50% for regional cerebral perfusion. The primary reason for use of deep hypothermic circulatory arrest was personal experience (75%) and/or opinion of experts (57%). Regional cerebral perfusion was most frequently endorsed for suggestive literature (71%) and personal experience (64%). A majority acknowledged that no definitive literature exists.
Currently, no data exist that justify the avoidance of less than 40 minutes of deep hypothermic circulatory arrest in favour of alternative strategies. Despite these findings, many institutions have altered their strategies of intraoperative management to avoid deep hypothermic circulatory arrest.
Post-operative management
A rapid and uneventful recovery from the Norwood (Stage 1) operation requires placement of an appropriate sized modified Blalock–Taussig shunt or shunt from the right ventricle to the pulmonary artery that will provide the necessary resistance to flow of blood into the pulmonary circulation. Historically, avoiding pulmonary over-circulation and compromise to systemic output was attempted with variable success by maintaining pulmonary vascular resistance with inspired carbon dioxide and/or avoidance of oxygen.Reference Jobes, Nicolson, Steven, Miller, Jacobs and Norwood46 Current strategies focus on reducing systemic vascular resistance to avoid pulmonary over-circulation and decrease after-load on the functionally univentricular heart.Reference Furck, Hansen, Uebing, Scheewe, Jung and Kramer47, Reference Tweddell, Hoffman and Mussatto48 In concert with this change in management, oxygen has also been found not to be detrimental and actually increases the saturation of both arterial and mixed venous oxygen.Reference Bradley, Atz and Simsic49 Thus, it is reasonable to eliminate the historical practice of suctioning the trachea without pre-oxygenation, which can be associated with sudden cardiac decompensation.
Approximately 50% of centres routinely leave all sternums open at the end of the Norwood (Stage 1) operation.Reference Wernovsky, Ghanayem and Ohye11 The open sternum is felt to provide haemodynamic stability, but does delay endotracheal extubation. Centres where elective sternal closure occurs at the end of the Norwood (Stage 1) operation move the stable patients towards earlier extubation. Infusions of muscle relaxant and high-dose fentanyl (10 micrograms per kilogram per hour) were popular in the late 1990s following demonstration that high-dose fentanyl blunted the pulmonary hypertensive response to suctioning.Reference Hickey, Hansen, Wessel, Lang, Jonas and Elixson50 This approach was popular during the time period when oxygen was avoided. These high doses of fentanyl resulted in hypotension that usually required the use of infusions of epinephrine. Many centres now use intermittent narcotics for control of pain and reserve infusions of narcotic and muscle relaxants for unstable patients or patients with open sternums.Reference Wernovsky, Ghanayem and Ohye11
Technical advancements in intensive care have benefitted this high-risk population of patients. Rapid or continuous clinical assessment is facilitated by
• “point of care” testing of blood gases,
• Near Infrared Spectroscopy,
• in-unit digital radiographs of the chest, and
• in-unit echocardiography.
In the absence of a clinical study, the routine use of post-operative heparinReference Wernovsky, Ghanayem and Ohye11, Reference O'Connor, Ravishankar and Ballweg52 and transitioning to aspirinReference Li, You and Berezny51 to maintain patency of the shunt appears to have reduced the incidence of thrombosis of the shunt to the degree that many clinicians feel that equipoise no longer exists for a randomised, placebo-controlled trial. The degree of anticoagulation and type of monitoring varies among centres.
Reports from single centresReference Ravishankar, Dominguez and Kreutzer53, Reference Allan, Thiagarajan, del Nido, Roth, Almodovar and Laussen54 and the database of the Extracorporeal Life Support Organization show a 50% early survival following the use of extracorporeal membrane oxygenation after the Norwood (Stage 1) operation. Many centres performing the Norwood (Stage 1) operations have rapid deployment extracorporeal membrane oxygenation, which is used in this population for
• hypoxaemia,
• arrhythmia,
• low output, and
• cardiopulmonary resuscitation.
Patients requiring extracorporeal membrane oxygenation for acute decompensation or occlusion of their shunt seem to fare better than those patients requiring extracorporeal membrane oxygenation for failure to separate from cardiopulmonary bypass.Reference Ravishankar, Dominguez and Kreutzer53, Reference Allan, Thiagarajan, del Nido, Roth, Almodovar and Laussen54
Implementation of peri-operative surgical, medical, and monitoring strategies over the last two decades have contributed to improved early inpatient survival. Despite encouraging early results, infants with shunt-dependent parallel circulation are at high risk for death before the second stage surgery. The interstage period is more fully described in the manuscript in this Supplement to Cardiology in the Young titled “Feeding, Growth, Nutrition, and Optimal Interstage Surveillance for Infants with Hypoplastic Left Heart Syndrome” by David A. Hehir, MD et al. Briefly, interstage mortality is reported at 2–20%.Reference Azakie, Merklinger and McCrindle9, Reference Ghanayem, Hoffman and Mussatto55 The risk of interstage death has been linked to anatomic diagnosis and residual or recurrent lesions including
• a restrictive atrial communication,
• obstruction of the aortic arch,
• obstructed flow in the shunt,
• distortion of the pulmonary artery,
• atrioventricular valvar insufficiency, and
• dysrhythmias.
Commonly acquired gastrointestinal or respiratory infections that lead to hypoxia, dehydration, and/or impaired systemic perfusion have also been implicated as aetiologies for interstage death. Hence, interstage management after the Norwood (Stage 1) operation warrants ongoing vigilance beyond the initial early post-operative period and requires continued assessments and collaboration among caregivers, including parents. To date, monitoring at home of saturation of oxygen and change in weight as a means to identify earlier the presence of anatomic lesions and intercurrent illness has been associated with improved interstage survival.Reference Furck, Uebing and Hansen6, Reference Ghanayem, Hoffman and Mussatto55
Summary
Advances in peri-operative and surgical strategies for infants with hypoplastic left heart syndrome have resulted in significantly improved survival. However, the challenging anatomy and unstable circulation leave these patients at risk for compromised cardiac function, worsened neurodevelopmental outcome, and limitations to their quality of life. Multi-centric clinical trials may contribute to significantly reducing the comorbidities related to the complex peri-operative and surgical management of this unique population of patients.