Congenital heart diseases (CHDs) are defined as the structural abnormalities of the heart or intra-thoracic vessels present at birth that have actual or potential functional significance. CHDs are the most common birth defects and are associated with significant morbidity and mortality.Reference Hoffman and Kaplan 1 , Reference Hoffman, Kaplan and Liberthson 2 Rheumatic heart disease is the sequel to acute rheumatic fever following Group A Streptococcus infection and is the most common acquired heart disease in developing countries.Reference Carapetis, Steer, Mulholland and Weber 3 This combination results in a high burden of childhood heart diseases in Africa.Reference Zühlke, Mirabel and Marijon 4 Namibia gained independence from apartheid South Africa in 1990. It is a vast country with land area 824,268 km2 and a sparse population of 2.3 million people, 44% of whom are children. 5 It is classified as an upper middle-income economy, however, there is gross income inequality with a Gini coefficient of 0.57. 6 Before 2007, there was no paediatric cardiac service in Namibia. Outpatient cardiac clinics were started by a visiting paediatric cardiologist in Windhoek in 2010. Limited local surgical programme catering mostly for adults and older children was commenced. With significant human resource constraints – no paediatric intensivists, critical care nurses, cardiac anaesthetists and cardiac surgeons – a centre for cardiac surgery for babies and small children under 20 kg had not been established. In response, in 2009 a public–private partnership was brokered between the Christian Barnard Memorial Hospital in Cape Town, South Africa and the Namibian Ministry of Health and Social Services. Funding for care is the responsibility of the Namibian government. Although the goal is to develop a fully functional and sustainable paediatric cardiac service in Namibia, in the interim patients are diagnosed and investigated in Windhoek by the single paediatric cardiologist at the country’s tertiary referral centre. Where appropriate, selected on merit, the infants and children weighing <20 kg, or >20 kg but with complex diseases are referred to South Africa for surgery and/or catheter interventions. This study describes the diagnoses, clinical characteristics, interventions, post-operative morbidity and mortality, and the follow-up of indigent, marginalised patients without health insurance referred to Cape Town between 2009 and 2015.
Methods
Study design and population
We performed a retrospective single-centre analysis of the 193 patients diagnosed, referred and managed by a single paediatric cardiologist between 2009 and 2015 from Windhoek Central Hospital to the Christian Barnard Memorial Hospital. Patient selection for surgery or intervention followed clinical and echocardiographic assessment and when relevant, cardiac catheterisation.
Ethics
The study was performed in accordance with the ethical standards described in the Declaration of Helsinki, with ethical approval from Institutional Review Boards at the University of Cape Town and the Ministry of Health and Social Services in Namibia. Parental consent had been obtained for surgery. We obtained a waiver of parental consent for this study.
Data collection
Data were captured from Windhoek Central Hospital and Christian Barnard Memorial Hospital registries using a standardised data collection form (Baseline Case Record Form – Appendix 1) and recorded in Research Electronic Data Capture (REDCap) database. Dependent and independent variables included demographic profile, primary diagnosis comorbid and pre-operative data, echocardiography findings, cardiac catheterisation, surgery, post-operative morbidity and mortality, and longitudinal follow-up outcomes. Patients were categorised using age and/or weight criteria and complexity estimated according to the presence of more than one lesion, associated pulmonary hypertension, or complex CHD. The modified version of the hierarchy of heart defects developed by the CONgenital COR Vitia (CONCOR) registry was used for the primary diagnosisReference van der Velde, Vriend, Mannens, Uiterwaal, Brand and Mulder 7 and assigned codes according to the International Classification of Diseases and Related Health Problems 10th revision coding system. 8 The Risk Adjustment for Congenital Heart Surgery (RACHS-1) modelReference Jenkins 9 , Reference Kang, Cole, Tsang, Elliott and de Leval 10 used here represents surgical complexity. Procedure nomenclature is assigned using the International Paediatric and Congenital Cardiac Code.Reference Franklin, Beland and Colan 11
Statistical analysis
Data were analysed using STATA 14.2 (StataCorp, 4905 Lakeway Dr, College Station, TX, United States of America). Continuous variables were expressed as means with SDs or medians with interquartile ranges as appropriate. Categorical variables were expressed as absolute number frequencies and percentages. Linear regression models and Cox regression models were used to assess the relationship between and risk of appropriate variables with survival, respectively.
Results
Demographics
A total of 193 patients were referred to the Cape Town centre for cardiac care. Patients originated from all regions of Namibia and all were indigenous to Namibia (Fig 1). Distances to the referral centre ranged from 200 to 800 km with 107 people (55%) referred from villages over 700 km from Windhoek Central Hospital. The ratio of female to male was 1.3:1. The median age for presentation with CHD and acquired heart disease categories were 12 months with interquartile range 5.0–60.0 and 210 months with interquartile range 180–276 months, respectively. The median age at referral to Christiaan Barnard Memorial Hospital was 32.8 months with interquartile range 11.1–101.3.
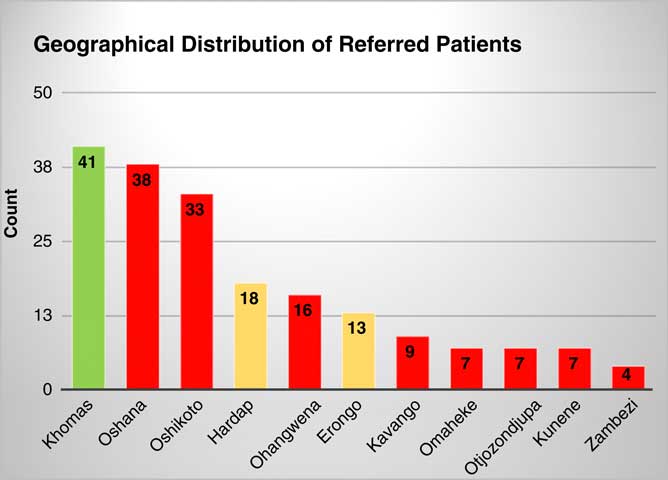
Figure 1 Geographical distribution of referred patients. (Green: referral centre, Orange: between 400 and 500 km, Red: further than 600 km.)
Baseline characteristics
Primary diagnosis
CHD accounted for 179 (93%) patients. “Simple” left to right shunts were the largest group, n=77 (39.9%), 52 of which were ventricular septal defects. Partial and complete atrioventricular septal defects were found in 17 (8%) patients. Complex cyanotic lesions, excluding tetralogy of Fallot, accounted for 29 (15%) patients. Obstructed left heart was found in seven patients (see Table 1). Only 14 (7.3%) patients had acquired heart diseases – rheumatic heart disease (n=13) and Takayasu arteritis (n=1). The predominant lesion in the rheumatic heart disease group was mitral stenosis (n=7, 50%), of which one patient had mixed mitral valve disease, one severe mitral and aortic regurgitation, and one isolated mitral regurgitation.
Table 1 Primary diagnosis, anatomical and physiological classification, CHD.
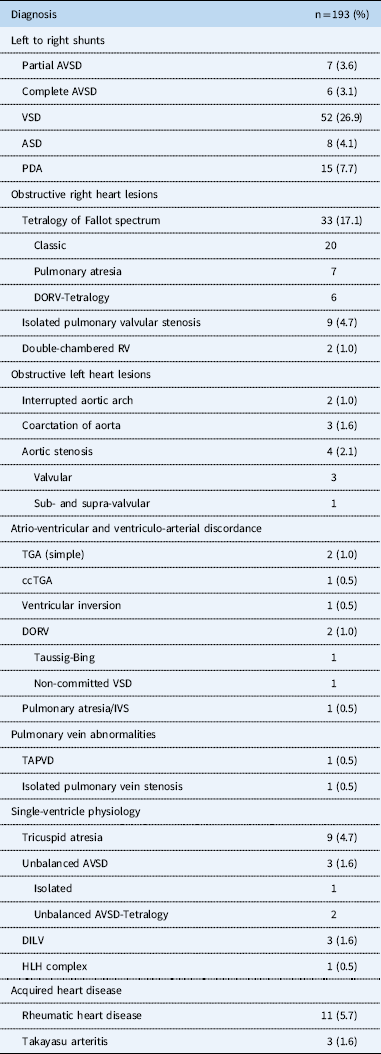
AVSD=atrioventricular septal defect; ASD=atrial septal defect; ccTGA=congenitally corrected transposition of the great arteries; DILV=double-inlet left ventricle; DORV=double-outlet right ventricle; HLH complex=hypoplastic left heart complex; IVS=intact ventricular septum; PDA=patent ductus arteriosus; RV=right ventricle; TAPVD=total anomalous pulmonary venous drainage; TGA=transposition of the great arteries; VSD=ventricular septal defect
Comorbidity
The number of patients with Trisomy 21 was 13 (6.7%). The 22q.11 micro-deletion was isolated in five patients (2.6%) but not all patients with CHD were screened for this gene defect. Heterotaxy was discovered in four (2.1%), Noonan’s syndrome was diagnosed in two patients (1.0%), congenital diaphragmatic hernia in three patients (1.6%), and one patient had the vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities association. Significant failure to thrive because of poor somatic growth ⩾3 SDs and below the mean for age and height was found in 76 patients (39.4%). In our cohort, 80 (41.5%) had clinical features and echocardiographic evidence of pulmonary hypertension. Almost three-quarters of the patients had a delay to surgery because of an existing respiratory infection; two patients were on respiratory support before surgery; and two patients with mitral stenosis because of rheumatic heart disease referred for percutaneous transcatheter mitral valve commissurotomy were in the third trimester of pregnancy.
Catheterisation
Diagnostic cardiac catheterisation
Cardiac catheterisation was performed in 89 patients (46.1%). The majority of patients, 62 (69.7%), were diagnostic/haemodynamic studies. Median overall age at catheterisation was 70.1 months with interquartile range of 17.6–155.4, median age for diagnostic catheterisation was 55.5 months with interquartile range 18.4–116.5 months, and median age for haemodynamic catheterisation was 36.7 months with interquartile range of 12.2–48.4 months. Catheterisation was performed in 30 (33.7%) patients with severe pulmonary hypertension to assess suitability for surgery. Of this group, three patients proved to have irreversible pulmonary hypertension secondary to pulmonary vascular disease and deemed inoperable. Catheterisation was done as routine inter-stage assessment in those undergoing staged single-ventricle palliation or to delineate anatomy for biventricular repair in 32 (36.0%).
Transcatheter interventions
There were 28 (30.3%) transcatheter interventions on 27 patients, including eight percutaneous mitral transcatheter commissurotomies and two radio-frequency ablations (see Fig 2). The remainder were for CHD interventions. There was one complication where an Amplatzer patent ductus arteriosus occluder device 1 was embolised into the left pulmonary artery. The device was removed and the duct ligated surgically.

Figure 2 Transcatheter interventions.
Surgery
The median age at presentation to Windhoek Central Hospital was 12 months with interquartile range of 5.0–47.5 months, however, the median age at the time of surgery was 28.4 months with interquartile range 11.1–78.0. In total, 156 cases were performed; surgery was elective in 142/156 (91.0%) cases. Of all patients, 27 (17.3%) who underwent surgery had palliative procedures. Of these 27 patients, 11 were for single-ventricle palliation – 10 Glenn shunts and one total cavopulmonary connection – six patients had a right-modified Blalock–Taussig shunt, nine had pulmonary artery bandings, and one had a central shunt.
A total of 129 (82.7%) patients had a full repair with 15 of these (11.6%) performed off cardiopulmonary bypass (see Fig 2). Those classified as “other” included three patients with double-outlet right ventricle, five patients undergoing right ventricular outflow tract reconstruction and/or pulmonary valve replacement, two patients with divided right ventricle and ventricular septal defect repair, and one each of left atrioventricular valve repair, tricuspid valve repair, divided right ventricle, ventricular septal defect and sinus of Valsalva repair, and ventricular septal defect/aortic valve repair (Fig 3). Risk stratification for surgical procedures (RACHS-1 categories) is illustrated in Table 2.

Figure 3 Cardiac surgery cases by procedure. ASD=atrial septal defect; AVSD=atrioventricular septal defect; AVR=aortic valve replacement; CoA=coarctation of the aorta; IAA=interrupted aortic arch; MVR=mitral valve replacement; PDA=patent ductus arteriosus; TOF=tetralogy of Fallot; VSD=ventricular septal defect.
Table 2 Surgical risk classification.

There were 11 re-operations (7.0%); four for haemodynamically significant residual defects, two in a child with pulmonary atresia, one with excessive pulmonary blood flow after systemic to pulmonary shunt, one with persistent ascites after right ventricular outflow tract reconstruction in tetralogy of Fallot with hypoplastic branches, and one for a residual ventricular septal defect after atrioventricular septal defect repair. However, two patients needed emergency revision: one for refractory bleeding and the other for systemic to pulmonary shunt thrombosis.
There were two babies with congenital diaphragmatic hernia and one with tracheoesophageal fistula who had those lesions repaired before cardiac surgery, and 12 patients with CHD were not offered surgery (Table 3).
Table 3 Patients not offered surgery.

* Lost to follow-up
ASD=atrial septal defect; AVSD=atrioventricular septal defect; AV=atrioventricular; IVC=inferior vena cava; LV=left ventricle; MAPCAs=medium-sized arterio-pulmonary collateral arteries; PDA=patent ductus arteriosus; PVRi=pulmonary venous resistance indexed; VSD=ventricular septal defect; WU=Woods Units
Outcome
Morbidity
Intra-operative morbidity
One intra-operative complication was documented. This patient sustained left coronary injury during a single-ventricle palliation with bilateral pulmonary artery augmentation. The coronary artery was repaired with successful re-vascularisation.
Post-operative morbidity
In a total of 156 patients, 80 (51.3%) had postoperative complications, of which 15 (9.6%) were a direct complication of cardiac surgery (Table 4). The median post-operative stay in intensive care was 5 days with inter-quartile range 3–7 days and the maximum being 106 days. Prolonged ICU stay, defined as 5 days after surgery, occurred in n=81 (51.9%).
Table 4 Postoperative complications.

Mortality
In total, there were eight deaths. Surgical mortality was 8/156 (5.1%, 95% confidence interval 2.2–9.8), of which five were early (<28 days) and three late deaths (Table 5). Of five early deaths, two occurred within 24–48 hours of surgery, two on day 5 and one each on days 20, 50, and 90, respectively. The 30-day mortality rate was 3.8 (95% confidence interval 1.4–8.2). Median age at death was 7.3 months with interquartile range of 6.2–22.5.
Table 5 Mortality.

ASD=atrial septal defect; AV=atrioventricular; AVSD=atrioventricular septal defect; DORV=double-outlet right ventricle; LAD=left anterior descending artery; MAPCAs=medium-sized arterio-pulmonary collateral arteries; PA=pulmonary artery; PDA=patent ductus arteriosus; RVOTO=right ventricular outflow tract obstruction; TGA=transposition of the great arteries; TOF=tetralogy of Fallot
Longitudinal follow-up rates and overall survival
A total of 42 patients (21.8%) were lost to follow-up, that is failed to attend a scheduled visit for 24 months from the last visit, over the period 1 December, 2009 to 31 December, 2016.
Discussion
The major findings of this study from Africa are that despite the distance to the referral centre – more than half of all patients referred for cardiac surgery and intervention reside more than 700 km from the only referral centre in Namibia – and their late presentation and referral, surgical therapy and/or intervention could be offered to the patients with good outcomes with an acceptable surgical mortality of 4.2% despite complex disease, significant comorbidity, and protracted post-operative intensive care. A high number of these patients, 21.8%, are lost to follow-up within 24 months.
Distance to care
In poor countries, long distances to care represent an additional access challenge. The majority of our patients live in the northern regions of Namibia in mostly rural, agrarian communities surviving through subsistence farming. If they are recognised as having cardiac disease, they are sent to the regional centre for paediatric services at the Oshakati Intermediate Hospital. This hospital is located in excess of 700 km from the national referral centre in Windhoek. The Ministry of Health and Social Services patient transport between Oshakati and Windhoek takes place by road once a week. Emergencies are transported by an air ambulance after lengthy procedural delays and at substantial extra direct cost. Poverty, distance to care, and poor transport infrastructure represent significant barriers to all levels of health care and of course to responsible follow-up. Recognising these barriers to care, regular outreach visits to the north of Namibia were instituted in 2013 to provide patient care and upgrade local knowledge and cardiology skills.
Late diagnosis and referral, complex disease, and significant comorbidity
The late presentation of patients with CHD to specialist care is primarily a consequence of poor early diagnosis and late recognition. This reflects poor knowledge of CHD in the peripheral referral regions of the country and the low penetrance of specialist paediatric services through the health system. Late detection has two significant results. First, that those patients with critical CHD (20% of all CHD)Reference Wren 12 who should have early surgery are never detected and die without treatment. The low numbers of babies with, for example, transposition of the great arteries or anomalous pulmonary venous connection in this cohort can be understood in this context. Second, by the time patients are diagnosed they have severe complications of their underlying disease. Pulmonary vascular disease secondary to severe pulmonary hypertension is the most common example and despite the preselection of patients by the cardiologist in Windhoek, a further three patients with pulmonary hypertension were deemed inoperable in Cape Town. The review does not reflect those many patients denied referral from Windhoek because of established pulmonary vascular disease. The problems posed by pulmonary hypertension are further reflected in the nine patients with post-operative pulmonary hypertensive crises. The higher age at presentation in the CHD group is also the reason for the high number of diagnostic cardiac catheterisations performed to assess the reversibility of pulmonary hypertension before surgery. Furthermore, adjunct multi-modality cardiac imaging, for example CT and MRI services are not available in Namibia. Although failure to thrive is often multi-factorial, particularly in countries with high levels of unemployment, poverty, and malnutrition, the high numbers with failure to thrive in the CHD cohort reflects the severity of their disease and a period of almost 22 months between presentation and referral for assessment. Pre-existing respiratory infection is a known risk factor for cardiac surgery.Reference Malviya, Voepel-Lewis and Siewert 13 A high number of children arrived for surgery with pre-existing respiratory disease, had in-hospital delay to operation and lengthy post-operative recovery.
The case-mix is diverse, predominantly CHD and predictable. The number of patients with rheumatic heart disease (n=13) was low which does not reflect the high prevalence of this preventable disease in Namibia.Reference Zühlke, Engel and Karthikeyan 14 , Reference Zühlke, Karthikeyan and Engel 15 Cardiac surgery was started at the Windhoek Central Hospital in October 2010 and through a similar period, the majority of patients around 200 reported cases with rheumatic heart disease needing valve surgeryReference Tangeni Auala, Henning du Toit and Nghaamwa 16 had surgery at Windhoek Central Hospital. Those with rheumatic heart disease sent to Cape Town, including children with weight under 20 kg and seven young women needing percutaneous transcatheter mitral valve commissurotomy for mitral stenosis when the technology known as Inoue percutaneous transcatheter mitral valve commissurotomy catheter was not available at Windhoek Central Hospital. Of the palliative operations, six patients had a right-modified Blalock–Taussig shunt, nine had pulmonary artery bandings, and one had a central shunt; these were performed as preliminary staging for future univentricular or biventricular repair and subsequently performed within the study period. In two patients, pulmonary artery banding was performed as final palliation owing to high-risk comorbidity.
Surgical mortality and length of intensive care
The low surgical mortality compares favourably with similar programmes in developing countries and international standards established by the Society of Cardiothoracic Surgeons.Reference Jenkins, Castaneda and Cherian 17 – Reference Yacoub, Hosny and Afifi 20 The likely causes of the post-operative deaths are revealing and suggest that in three patients the risk associated with restrictive right ventricular pathophysiology was not fully appreciated pre-operatively. Two patients with complex atrioventricular septal defects also had abnormal right ventricle pathology. Pre-operative pulmonary hypertension was neither associated with mortality or length of time in ICU suggesting appropriate pre-selection. The length of post-operative intensive care correlates with mortality. Pre-operative respiratory infection was common (75%) but neither this nor failure to thrive was a risk factor for death even though both factors were associated with the prolonged post-operative ICU stay.
Although not directly comparable, a review of surgical outcomes between 2010 and 2015 on a cohort of 200 patients with rheumatic heart disease warranting valvular repair or replacement in the Cardiac Unit in Windhoek concluded a surgical mortality rate of 5.5% with a 17% mortality over 34 months. Children <18 years old constituted 26.3% of this cohort.Reference Tangeni Auala, Henning du Toit and Nghaamwa 16
Loss to follow-up and after-care
Post-operative care and surveillance is a determinant of long-term outcome. The outreach paediatric cardiology service to the north of the country was started in 2013 partly to address this challenge. Although it has improved, patient “connection” with primary health facilities and with specialist cardiac care is poor. The lengthy distances to the referral centres, poor transport infrastructure, and the fact that many of our families live in abject poverty further contribute to the problem. Many families simply cannot afford private transport to the hospitals or primary care services to sustain their follow-up care. There is no electronic medical record and no national database for patients within the system, so case-finding and patient tracking remain problematic. Despite the health service challenges, the combination of a patient registry, outreach programme, and the continuity of care provided by a single cardiologist have contributed to 80% of patients remaining within follow-up, which is favourable compared with reports from elsewhere in Africa.Reference Zühlke, Karthikeyan and Engel 15 , Reference Damasceno, Mayosi and Sani 21 However, the loss to follow-up rate of 20% is still high and suggests that our mortality statistics underestimate late post-operative deaths.
Implications for health policy, practice, and research
The Namibian Children’s Heart Project is a humanitarian programme aimed to provide cardiac surgery and intervention for indigent Namibians who are unable to access such services in Namibia. It is a South–South collaboration and a unique funding model on the African continent with national government purchasing services from private sector providers in a neighbouring country.
This public–private partnership is an interim measure while services are slowly being developed and strengthened and people are getting trained to develop capacity. It was developed in response to the need in Namibia. In addition, in South Africa, the only country in the region where paediatric cardiac surgery is performed, surgery in its public hospitals for patients from other countries is prohibited. In these circumstances, this public–private partnership, with the Ministry of Health and Social Services purchasing services from the CBMH was the only solution. Furthermore, the total population of Namibia is 2.4 million and paediatric surgical centres are recommended at a rate of 1 for every 5 million people. Our goal is ambitious but with the efforts being made to strengthen capacity, it is, we believe, attainable.
Although the Namibian Children’s Heart Project has brought relief to almost 200 patients with satisfactory results by December 2015, these patients should be able to get the care they require in Namibia. There is overwhelming general agreement that the birth prevalence of CHD is 8 per 1000 live births.Reference Hoffman, Kaplan and Liberthson 2 , Reference Hoffman 22 Applying this to Namibia, with annual births per annum of 60,000, 23 there are 480 babies born every year with CHD. Of these 40% will need heart surgery, and 20% who have critical CHD require it in infancy.Reference Hoffman and Kaplan 1 , Reference Wren 12 , Reference Hoosen, Cilliers and Hugo-Hamman 24 Without addressing the backlog, 200 patients over 6 years is not enough, and the Ministry of Health and Social Services should be planning heart surgery for at least 200 patients per year in the country.
It is our objective to develop a comprehensive, sustainable, and self-sufficient paediatric cardiac surgical service in Namibia. The challenges are both human resource-based and infrastructural. The minimum specialist requirements are a paediatric cardiac surgeon with sufficient training and recognised expertise in CHD, anaesthetists trained in paediatric cardiac care, a paediatric intensive care specialist, and a well-trained cadre of paediatric cardiac intensive care nurses. To address this challenge, the Namibian government funded training for a paediatric cardiologist and a paediatric cardiac surgeon at the University of Cape Town and Red Cross War Memorial Children Hospital in South Africa. Both of whom return to Namibia in December 2018. In a country without paediatric intensive care, construction of such a facility is a stated priority for national government. Only with this facility, appropriate financial resources from government for heart surgery in children and a collective and cooperative effort to upgrade nurse ICU skills for babies and children will we be able to offer safe surgery within Namibia. Implementation requires strict adherence to established quality improvement protocols, with proposed participation in the International Quality Improvement Collaborative.Reference Jenkins 9 , Reference Jenkins, Castaneda and Cherian 17 Diagnostic services within the health department need to improve as without this, the sick new-born with critical CHD will remain invisible. 25 , Reference Zheleva and Atwood 26 That too is a challenging task. The National Ministerial Outreach programme should be expanded and diagnostic echocardiogram technology and skills need to be provided at intermediate hospitals across the country. Compulsory oximetry screening according to available guidelines should be considered in neonatal units to improve detection yields. Underpinning these tasks are the data which demand a national database of heart disease in children.
Conclusion
We have demonstrated satisfactory outcomes for those fortunate to be referred despite health services constraints, adverse characteristics of multiple lesions, and complexity associated with late presentation, and the 30-day mortality rate is only 3.2%. The cohort reports only those who travelled to Cape Town and does not address the challenge of the many patients who were referred but died or became inoperable, whilst waiting. This study highlights health service deficiencies needing improvement and a commitment from the team to achieve safe surgery for babies and children within a sustainable national programme in Namibia.
Acknowledgements
The Harold and Ethel Pupkewitz Heart Foundation has supported this study. Mr Harold Pupkewitz, through the “Child Survival, Protection and Development Foundation”, provided funding for the first nine patients. Funding for setting up the database was provided by the Children’s Heart Disease Research Unit at Red Cross War Memorial Children’s Hospital. The authors wish to thank Mr Wisdom Basera for statistical assistance. The Permanent Secretary of the Ministry of Health in Namibia has approved this publication.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors. L.J.Z. receives funding from the Medtronic Foundation through support to RHD Action. L.J.Z. is also supported by the National Research Foundation of South Africa (NRFSA) and the Medical Research Council of South Africa (MRC).
Conflicts of Interest
The authors declare no conflicts of interest.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (Human Research Ethics Committee University of Cape Town) and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees (Human Research Ethics Committee Reference number 762/2016) and the Ministry of Health and Social Services in Namibia (17/3/3).This work is in partial submission of the MPhil (Paediatric Cardiology) degree at the University of Cape Town by Dr Shidhika.










