During fetal development, both the atrioventricular valve leaflets and the chordae tendinae are derived from the endocardial cushions. From the trabecular muscle of the left ventricle arise compacting columns that form a muscular ridge. The anterior and posterior part of this ridge will become the papillary muscles by a process called delamination, which consists of a gradual loosening of muscle. Therefore, in normal patients, papillary muscles are organised as two discrete groups of papillary muscles, which arise from the apical two-thirds of the left ventricular wall. The tendinous cords extend from their tips. In postnatal life, papillary muscles hold a superolateral and inferomedial position.Reference Séguéla PE and Acar 1 We have chosen to use this consistent and anatomically correct terminology, rather than the traditional misleading terms – anterolateral and posteromedial.Reference Anderson, Razavi and Taylor 2
In necropsy studies, considerable anatomical variability of the papillary muscles has been observed. Each papillary muscle may be viewed as a major trunk from which a variable number of heads or fingers project. According to Roberts and Cohen,Reference Roberts and Cohen 3 the superolateral papillary muscle usually consists of a single major muscle group (75% of cases), whereas the inferomedial papillary muscle often consists of two or three major muscle groups (65%). The base-to-apex lengths of the papillary muscles also vary considerably.
In normal left ventricles, papillary muscles arise at the junction of the middle and lower thirds of the left ventricle. If malpositioned, they may arise from the upper third of the ventricular wall, causing inflow stenosis.Reference Roberts and Cohen 3 The most frequent congenital papillary muscle malformation is the occurrence of only a single muscle, which may be the most common cause of congenital mitral stenosis. In addition to this, asymmetric papillary muscles can develop when one of the two do not correctly delaminate from the ventricular wall.Reference Séguéla PE and Acar 1 An accessory papillary muscle is also a common variant but is usually of no functional significance.Reference Roberts and Cohen 3 A true parachute mitral valve is a relatively rare abnormality, resulting from abnormal compaction of the left ventricular myocardium. In this article, we describe the use of cardiac magnetic resonance to assess papillary muscle morphology and demonstrate how this varies between normal, borderline, and hypoplastic left ventricles.
Materials and methods
This was a single-institution, retrospective, observational study. Institutional Review Board approval was obtained before commencing the study.
Cardiac magnetic resonance is utilised in a wide range of patients to assess the anatomy and function of the heart and the great vessels. All cardiac magnetic resonance scans were performed on a 1.5-T Achieva clinical magnetic resonance scanner (Philips Healthcare, Best, The Netherlands). General anaesthesia was administered when required according to patient age and cooperation.
Patients with borderline left ventricles have a cardiac magnetic resonance as part of their diagnostic study before undergoing surgery and during their follow-up according to the institutional protocol. A selection of standard short-axis cines and three-dimensional, whole-heart, steady-state, free-precession sequencesReference Hussain, Lossnitzer and Bellsham-Revell 4 , Reference Heathfield, Hussain and Qureshi 5 were used to assess the position of the papillary muscles, their length ratio – that is, length of papillary muscles/length of the left ventricle – the angle between both papillary muscles, the number of major muscle groups, and the characteristics of their insertion.
A whole-heart, three-dimensional, steady-state, free-precession sequence was acquired in a sagittal orientation (repetition time ms/echo time ms, 3.4/1.7; flip angle, 70°; number of slices, 60–120; isotropic resolution, 1–1.5 mm3; acquisition window, 60–75 ms). Short-axis cines were acquired using a standard, retrospective, two-dimensional, steady-state free-precession sequence. Imaging parameters were as follows: repetition time/echo time: 3.0 ms/1.5 ms, flip angle 60°, 30 cardiac phases per average heart beat, field of view 280–400 mm, matrix size 172–196, slice thickness 6–10 mm, no intersection gap, sensitivity encoding for spatial under-sampling (factor 2), 12–16 slices to cover the ventricles from the apex to the base – that is, six to eight breath holds.
The length ratio of the papillary muscles was analysed in each patient using three-dimensional, steady-state, free-precession. A four-chamber view on multiplanar re-format allowed the measurement of the length of the left ventricle from the mitral annulus to the apex. The length of the papillary muscles was estimated parallel to the axis of the left ventricle scrolling from the view previously described (Fig 1).

Figure 1 The length of the papillary muscles was estimated parallel to the axis of the left ventricle, scrolling from a four-chamber view on a multiplanar re-format.
Patients were divided as having a discrete or a broad insertion depending on the attachment to the ventricle of the bellies defining them. When attaching immediately adjacent to each other to the ventricular wall or when defined by a single belly, papillary muscles where considered to have a narrow insertion; when inserting as separated bellies far from each other, they were considered to have a broad insertion (Fig 2, Supplementary videos 1 and 2).

Figure 2 ( a ) Narrow insertion of the bellies in the superolateral support (*) in a normal-sized left ventricle (LV). ( b ) Broad insertion of the bellies in the superolateral support (★) in a borderline LV.
When having a discrete insertion, the angle between major muscle groups was measured. In order to perform the measurement, the centre point of the muscles group was taken as a reference, and a line was drawn through the angle subtended at the centre of the left ventricle between the centre points of the papillary muscle group insertion points (Fig 3).

Figure 3 The centre point of the muscle group was taken as a reference, and a line was drawn through the angle subtended at the centre of the left ventricle between the centre points of the papillary muscle insertions.
Patients were divided into three groups – normal, borderline, and hypoplastic – according to left ventricle volume indexed by body surface area. Patients were included in the hypoplastic group if the end-diastolic volume was ⩽13 ml/m2 or ⩽20 ml/m2 with definite features that precluded for biventricular repair, such as mitral atresia, severe mitral hypoplasia, and aortic atresia with intact ventricular septum.Reference Grosse-Wortmann, Yun and Al-Radi 6 , Reference Minich, Tani, Hawkins and Shaddy 7
In borderline cases, biventricular repair was not ruled out at initial surgery. A hybrid procedure, consisting of pulmonary artery banding and patent arterial duct stenting, was performed in all patients with either hypoplastic or borderline left ventricles.
Statistical analysis was performed using SPSS software (version 19, 2010; SPSS, Chicago, Illinois, United States of America). Analysis of variance test was used to compare the mean length ratio for papillary muscles, age, and angle between papillary muscles. The Bonferroni correction was applied for post hoc testing. The number of papillary muscles and the characteristics of their insertion were analysed using Fisher’s exact test. The splitting of papillary muscles between groups was analysed using a Kruskal–Wallis test.
Results
Patients
A total of 30 consecutive patients with normal-sized left ventricles, 22 with borderline, and 13 with hypoplastic left ventricles were included in the present study. Their median age was 5.36 years (with a range from 1 month to 20 years). There were 26 female and 39 male patients.
Diagnosis
Among patients with normal-sized left ventricles, five had mild valvar CHD or mild pulmonary artery stenosis, 18 had a repaired biventricular CHD without left-sided lesions, and five were referred for screening and had a normal heart.
Borderline left ventricles comprised a heterogeneous group of patients with obstruction of the left heart at different levels.
Among patients with hypoplastic left heart syndrome, three of them had aortic atresia, one of them with associated mitral stenosis; five patients had ventricular septal defect as an associated feature.
Associated diagnostic features for borderline and hypoplastic left ventricles are summarised in Tables 1 and 2.
Table 1 Associated features in patients with borderline left ventricles.

AAH=aortic arch hypoplasia; ASD=atrial septal defect; AoV=aortic valve; EFE=endocardial fibroelastosis; IAA=interrupted aortic arch; PDA=patent ductus arteriosus; uAVSD=unbalanced atrioventricular septal defect; VSD=ventricular septal defect
* Same patient
** 1 patient is included in both groups
Table 2 Associated features in patients with hypoplastic left heart syndrome (HLHS).

AAH=aortic arch hypoplasia; CS=coronary sinus; DORV=double-outlet right ventricle; LA=left atrium; VSD=ventricular septal defect
Major muscle groups: number and tendency to split
The number of major muscle groups was found to be significantly different between groups (Fig 4) (Fisher exact test, p<0.001). All patients included in the normal-sized group had two discrete major muscle groups; however, four (18%) borderline and six hypoplastic (46%) left ventricle cases had a single papillary muscle (Table 3). Interestingly, the superolateral support was the absent muscle in 90% of patients with a single papillary muscle.
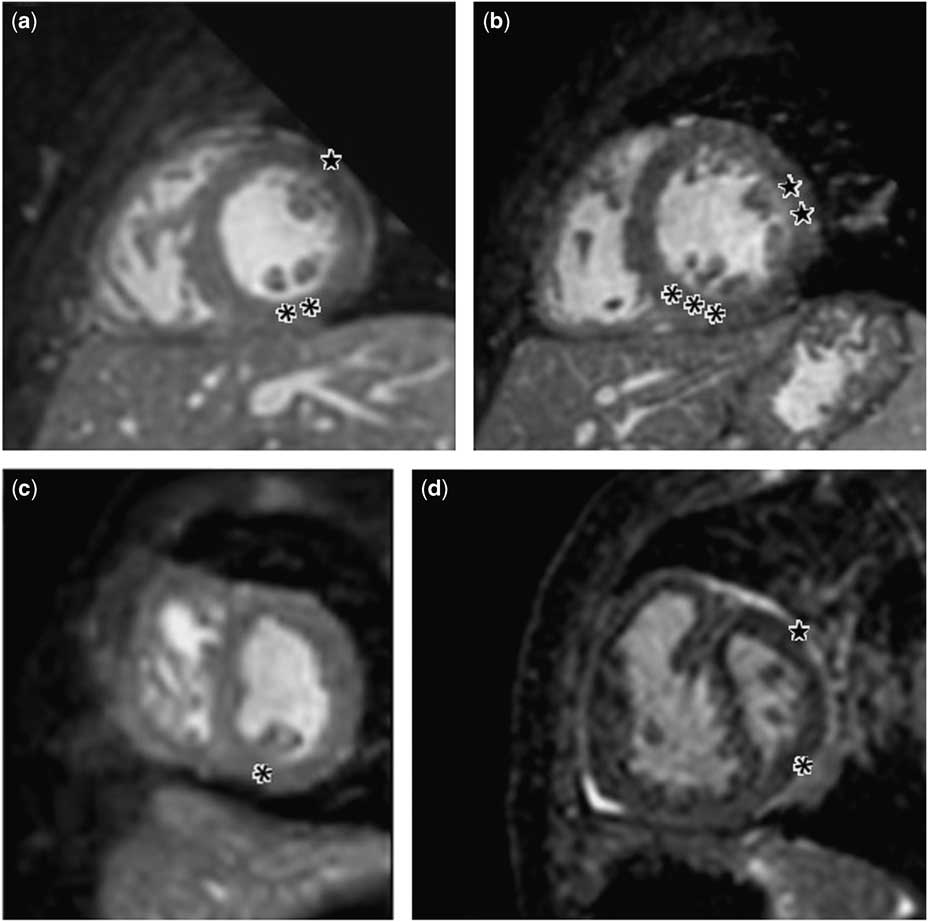
Figure 4 ( a ) Most common papillary muscle arrangement in normal-sized left ventricle (LV): **inferomedial support defined by two bellies; ★ superolateral support defined by one. ( b ) Normal-sized LV showing several bellies defining each group (★★ superolateral support showing two bellies, ***inferomedial group showing three). ( c ) Single papillary muscle in borderline LV. ( d ) Papillary muscles defined by one belly in the superolateral (★) and inferomedial support in hypoplastic left heart syndrome (*).
Table 3 Number of patients with a single papillary muscle for each group and position of the remaining muscle.

SL=superolateral; IM=inferomedial.
Splitting of papillary muscles was a frequent finding. In each group and for each papillary muscle, splitting of the muscle into two to four bellies was observed (Fig 2). The number of bellies for each major muscle group (Table 4) was compared between normal-sized, borderline, and hypoplastic ventricles applying a Kruskal–Wallis test. In the inferomedial support, the number of bellies ranged from 1 to 3 in normal-sized ventricles and showed a mode of 2. In borderline ventricles, it ranged from 1 to 4 (mode=1), and in hypoplastic ones the inferomedial support was defined by one or two bellies when present (mode=1). A significant difference among groups was found regarding this support (Kruskal–Wallis test, p=0.023).
Table 4 Number of bellies in the superolateral and inferomedial support among groups.

The superolateral support showed one to three bellies in normal-sized and borderline ventricles when present (mode=1). In hypoplastic cases, the number of bellies was always 1 if present. No difference was detected when comparing the number of bellies of the superolateral papillary muscle between groups (Kruskal–Wallis test, p=0.211).
Overall, the inferomedial papillary muscle often split into more bellies (mode=2) than the superolateral muscle (mode=1; p=0.013 by Wilcoxon’s signed-rank test).
Insertion of pedicles
Insertion of papillary muscles was described as either narrow or wide for both the superolateral and the inferomedial muscles (Fig 2).
A narrow insertion with fused pedicles was observed in all 30 normal-sized left ventricles for both papillary muscles, whereas in borderline and hypoplastic cases the inferomedial muscle had a broad insertion in 17 and 8% of the patients, respectively. A significant difference was found when comparing this width of inferomedial insertion among groups (Fisher’s exact test: p=0.023). The superolateral papillary muscle showed a wide pedicle in 10% of cases with borderline left ventricles. This insertion was narrow in all cases with a hypoplastic or normal left ventricle (Fisher’s exact test comparing across groups: p=0.211).
Length ratio of papillary muscles
The mean length ratio of the superolateral muscle was significantly different across groups (borderline: 0.39±0.07; hypoplastic: 0.36±0.1; normal: 0.46±0.08; analysis of variance, p=0.009). Post hoc testing with Bonferroni’s correction showed significant differences in comparison with hypoplastic versus normal (p=0.035) and borderline versus normal (p=0.044).
In contrast, when analysing the mean length ratio of the inferomedial support, no significant difference was detected among patients from different groups (mean length ratio 0.42±0.09 in normal, 0.38±0.07 in borderline, and 0.39±0.22 in hypoplastic left ventricles; analysis of variance p=0.39).
Angle between major muscle groups
This angle was found to be similar in the three groups analysed (mean angle 113°±17° in normal, 111°±51° in borderline and 114°±57° in hypoplastic ones, analysis of variance p=0.99). Of note, the variance of the angle was much higher in borderline and hypoplastic ventricles compared with normal.
Biventricular versus univentricular repair in patients with borderline left ventricles
Patients with borderline left ventricles were separately analysed according to the type of surgical repair they had undergone. Age, length ratio of papillary muscles, angle between major muscle groups, insertion of pedicles, and end-systolic and end-diastolic volumes were compared. No significant differences were found for any of the features analysed.
Discussion
The mitral valve apparatus is assuming great importance in patients with borderline size of the left ventricle, as this structure may impact on single-ventricle versus biventricular repair. Furthermore, the introduction of the hybrid procedure as the first step in surgery for patients with borderline left ventricles provides the opportunity to decide whether single or biventricular repair is the best option for a certain patient at a later stage. This allows for more comprehensive surgery for left ventricle rehabilitation at a later age and at significantly larger size.Reference Quinonez and Del Nido 8 – Reference Nassar, Narayan and Nyman 10
In our study, a consecutive group of patients with hypoplastic left heart syndrome and borderline cases was included. All the patients were initially managed with a hybrid procedure. The cut-off dimension for end-diastolic volume has always been controversial. The potential of the left ventricle to grow after surgery is well established.Reference Minich, Tani, Hawkins and Shaddy 7 , Reference Corno 11 Minich et al performed two-dimensional and Doppler echocardiography to assess the possibility of left ventricular growth after birth in patients with hypoplastic left ventricles. They selected seven newborns from a cohort of 68 and described the minimum end-diastolic volume for biventricular repair as ⩾10 ml/m2 using echocardiographic measurements.Reference Minich, Tani, Hawkins and Shaddy 7 Different publications have reported a left ventricular end-diastolic volume of <20 ml/m2 as a risk factor for death in patients with borderline left ventricle.Reference Corno 11 , Reference Hammon, Lupinetti and Maples 12 , Reference Parsons, Moreau, Graham, Johns and Boucek 13 In this study, patients were included in the hypoplastic group if the end-diastolic volume was ⩽13 ml/m2 by cardiac magnetic resonance or ⩽20 ml/m2 if definite features that precluded for biventricular repair such us mitral atresia or severe mitral hypoplasia and aortic atresia with intact ventricular septum were diagnosed.
It was observed that a single papillary muscle was a common anomaly in patients with small left ventricle (18% in borderline left ventricle and 46% in hypoplastic cases in our series). The missing support is usually the superolateral type. When present, however, the superolateral support was significantly shorter in smaller left ventricles compared with normal. No difference in length ratio was found when comparing the inferomedial support.
Overall, the inferomedial papillary muscle often split into more bellies (mode=2) than the superolateral muscle (mode=1; p=0.013 by Wilcoxon’s signed-rank test). Furthermore, a wide insertion of either one or both supports is a common finding in small left ventricles and it is more often seen with the inferomedial papillary muscle.
The approach of papillary muscles during mitral stenosis relief and primary left ventricle rehabilitation has been described by del Nido et al.Reference Quinonez and Del Nido 8 , Reference Emani, Bacha and McElhinney 9 During surgery, patients with borderline left ventricles may undergo separation, splitting, of papillary muscles and abnormal attachments of papillary muscles to the septum or the left ventricle free wall as part of the relief of the left inflow tract obstruction. The description given by our cohort helps in understanding the morphological approach required – for example, given the patterns observed, lengthening of the superolateral papillary muscle chordae and splitting of the inferomedial muscle may be appropriate. At the very least, the described approach to defining morphology enables the experienced surgeon to plan his procedure in advance.
Electrocardiogram-gated three dimensional of the whole heart is a useful MRI sequence to assess the great vessels, the coronary arteries, and the cardiac chambers in patients with complex CHD including hypoplastic left heart syndrome.Reference Hussain, Lossnitzer and Bellsham-Revell 4 , Reference Heathfield, Hussain and Qureshi 5 , Reference Uribe, Hussain and Valverde 14 In this study, a combination of short-axis cine and three-dimensional whole heart demonstrated the anatomy of papillary muscles in all patients. These sequences are already a part of the MRI protocol in our centre in the assessment of patients with hypoplastic left heart syndrome before undergoing surgery and during follow-up. In their article, del Nido et al define MRI as essential in the imaging of patients with borderline left-heart structures who are considered candidates for left ventricle rehabilitation.Reference Emani, Bacha and McElhinney 9 Cardiac magnetic resonance also allows for the accurate evaluation of left ventricular volumes in this cohort to further empower decision making regarding suitability for biventricular repair.Reference Grosse-Wortmann, Yun and Al-Radi 6 The versatility of cardiac magnetic resonance imaging also allows for the detection of clinically important endomyocardial fibrosis.Reference Tworetzky, del Nido, Powell, Marshall, Lock and Geva 15
Even if cardiac magnetic resonance is the ideal technique to assess ventricular volumes, current resolution does not allow an accurate definition of the attachments of the mitral leaflets to the papillary muscles through the chordae. In recent publications, these characteristics have been described by using three-dimensional echocardiography. Rice et al, assert the utility of three-dimensional echo when assessing the chordal apparatus and its relationship to the papillary muscles. Their publication shows the importance of three-dimensional imaging to demonstrate the extent of the papillary muscle and to describe the subvalvar apparatus in situations such as the absence of chordae and loss of interchordal spaces that cannot be projected on two-dimensional echocardiographic images.Reference Rice and Simpson 16 Another recent study by Rim et al on parachute mitral valve demonstrated a strong correlation between the reduction of mitral orifice size and the degree of asymmetry of the papillary muscle location. Moreover, there was a considerable reduction in leaflet coaptation and abnormal leaflet deformation corresponding to the anomalous location of the papillary muscle tips;Reference Rim, McPherson and Kim 17 three-dimensional echocardiography therefore has a complementary role in the assessment of patients with borderline or hypoplastic left ventricles, and this is the patients of ongoing clinical research.
As a limitation to this study, the age of patients included in the different groups was found to be significantly different. The reason for this fact is that, owing to the young age of children undergoing cardiac magnetic resonance when suffering from hypoplastic left heart syndrome and its variants, it was difficult to achieve age match with normal controls. Therefore, children included in the normal group were older than those with borderline or hypoplastic left ventricles. In order to neutralise this limitation, all relevant quantitative values were indexed to heart size or body surface area. Although healthy patients would have been optimal for this project, paediatric scans rely on the use of general anaesthesia for the acquisition of images. Therefore, only clinical studies have been used with no volunteer inclusion; however, our findings for our control group in terms of papillary muscle morphology are similar to autopsy publications of normal hearts.Reference Roberts and Cohen 3
No morphological differences of the papillary muscles were found within this series when comparing children with borderline left ventricle undergoing successful biventricular repair against those undergoing single-ventricle surgical palliation. Given the small number of cases with biventricular repair included in the present study, significance may be reached with a bigger sample.
This study describes papillary muscle morphology by using cardiac magnetic resonance in hypoplastic and borderline left ventricles. It emphasises the importance of taking into account the anatomy of the papillary muscles when assessing the mitral valve, subvalvar apparatus, and the size of the left ventricles.
There is a tendency for the superolateral papillary muscle to be shorter or absent in small left ventricles, and the inferomedial support is often split into several bellies and sometimes shows a broad insertion. Although no differences were found between patients with borderline left ventricles undergoing biventricular repair and those following a single-ventricle route, this might be related to the small population size included in the present study.
Acknowledgements
None.
Financial Support
This study was supported by the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. The Division of Biomedical Engineering & Cardiovascular Imaging is part of the Centre of Excellence in Medical Engineering and is funded by the Welcome Trust and EPSRC (grant number WT 088641/Z/09/Z). King’s College London is a British Heart Foundation centre of excellence funded by the British Heart Foundation award RE/08/003.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Ethical Standards
All procedures performed in studies involving human patients were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study includes anonymised retrospective data only. For this study, specific formal consent was not required (Research & Development registration number: RJ115/N249).
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951117000439










