A low state of cardiac output occurs in up to one-quarter of children after repair of congenitally malformed hearts. It carries significant morbidity and mortality, often coupled with a prolonged stay in intensive care.Reference Wernovsky, Wypij and Jonas1 Early identification and management of the low cardiac output state, therefore, is essential in the postoperative care of these patients. It is well recognised, however, that the ability of clinicians to estimate cardiac index from physical examination and clinical and laboratory data is poor when compared to invasive measurement.Reference Tibby, Hatherill, Marsh and Murdoch2 Mixed venous saturation of oxygen has for some time been used as a tool to assess the adequacy of systemic delivery of oxygen. In children after cardiac operations, mixed venous saturation of oxygen and central venous saturation of oxygen are increasingly being used as surrogates for systemic delivery of oxygen and cardiac output. In this review, we consider the role of measurement of venous saturation of oxygen in the assessment of the child in intensive care after cardiac surgery.
The derivation of cardiac output
According to the Fick equation (Text Box 1), cardiac output is the ratio of consumption of oxygen to the arteriovenous difference in the content of oxygen. The content of oxygen in the blood refers to the sum of oxygen which is haemoglobin-bound, namely oxyhaemoglobin, and that which is dissolved in the plasma. When the consumption of oxygen remains constant, along with the concentration of haemoglobin, cardiac output is proportional to the difference in arterial and mixed venous saturations of oxygen, which in turn reflects the extraction of oxygen by the tissues. A fall in mixed venous saturation of oxygen, in the setting of constant arterial saturation and haemoglobin, therefore, implies either an increase in consumption by the tissues, or a fall in cardiac output.
Box 1 The Fick Equation
The application of mixed venous saturation of oxygen to estimate cardiac output
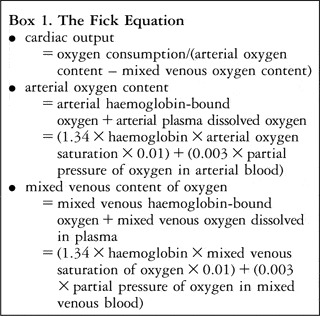
There are a number of inaccuracies associated with the use of mixed venous saturation of oxygen as a surrogate for cardiac output, which are largely related to the assumptions which must be made in order to apply this approach. First, the assumption that the concentration of haemoglobin is constant may be erroneous. While the level of haemoglobin is regularly measured early after cardiac surgery, it is not constantly monitored, and is subject to significant shifts of fluid and fluctuation due to loss of blood. These phenomenons are relatively common early after cardiac surgery, which is obviously the period of greatest interest. The second important, and incorrect, assumption is that consumption of oxygen by the tissues remains constant early after cardiac surgery. Consumption of oxygen is, in fact, very unpredictable. It fluctuates with changes in medication, in particular with use of inotropes and sedation, and with the constantly changing physiological environment early after surgery, especially with changes in body temperature.Reference Jai, Bush, Schulze-Neick, Penny, Redington and Shekerdemian3–Reference Li, Schulze-Neick and Lincoln5 Third, the relationship between change in mixed venous saturation of oxygen and cardiac output is non-linear, even at constant arterial saturations of oxygen (Fig. 1). As a result, a given decrease in mixed venous saturation of oxygen may accompany a much larger decrease in cardiac output.Reference Tibby and Murdoch6 Fourth, in circumstances where arterial saturation of oxygen is low, such as lung disease or in the presence of an intra-cardiac right-to-left shunt, absolute venous saturations must be interpreted in light of the baseline arterial saturation. In this situation the arterial-venous oxygen difference, or the ratio of extraction of oxygen, which is the consumption of oxygen by the tissues divided by the delivery of oxygen, are of greater relevance (Text Box 2).Reference Tibby and Murdoch6 Finally, the measurement of mixed venous saturation of oxygen requires a means of sampling from an appropriate site. In patients without an intracardiac left-to-right shunt, this may be from the pulmonary arteries. Post-operative cardiac patients may have catheters sited in the pulmonary arteries at the time of surgery for monitoring of pressure, and this can also serve the purpose of sampling mixed venous blood. The frequency with which such are now inserted, however, is decreasing. In these patients, furthermore, the utility of this site for estimation of mixed venous saturation of oxygen is often limited by intracardiac left-to-right shunting through a residual interatrial or interventricular communication. Although a trivial septal defect may be haemodynamically insignificant, such a shunt would render the pulmonary arterial sample useless in terms of accurately representing mixed venous blood.
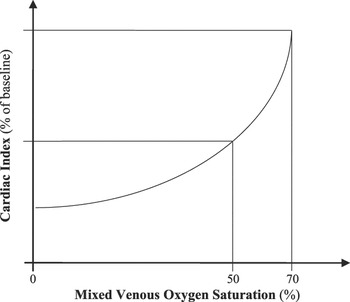
Figure 1 The graph demonstrates the non-linear relationship of cardiac index and mixed venous saturation of oxygen. For example, a fall by 20% in mixed venous saturation corresponds with a reduction in cardiac output of greater than 40%.Reference Li, Schulze-Neick and Lincoln5
Box 2 Arterio-venous oxygen difference and the oxygen extraction ratio

The utility of central venous saturation of oxygen as a predictor of mixed venous saturation of oxygen in healthy individuals
The use of central venous saturation of oxygen as a surrogate for mixed venous saturation of oxygen has been widely investigated in adults, but not in children. When assessing the potential utility of superior and inferior caval venous saturations as measures of mixed venous saturation of oxygen, a number of considerations must be made. The most important of these is the baseline difference in saturations between these two sites, which is related mainly to the venous return from the renal bed and hepatic portal system being more highly saturated than that from other tissues. As a result, superior caval venous saturation is 2–3% lower than the true mixed venous saturation in healthy adults (Fig. 2).Reference Nelson7, Reference Marx and Reinhart8 Interestingly, mathematical models have been developed to estimate true mixed venous saturation of oxygen from measurements of superior and inferior caval venous saturations. An estimate with a high degree of accuracy can be obtained using the following equation:
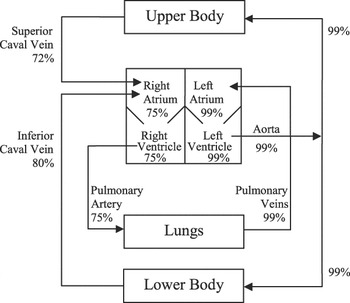
Figure 2 Arterial and venous saturation of oxygen in various vascular regions, in healthy individuals with septated hearts.Reference Nelson7

The utility of central venous saturation of oxygen as a predictor of mixed venous saturation of oxygen in shocked patients
In shock, where systemic balance of oxygen is typically jeopardised, the relationship between superior caval venous saturation and mixed venous saturation reverses, such that superior caval venous saturation is higher than mixed venous saturation, with the reported difference ranging from 5–18%.Reference Marx and Reinhart8, Reference Rivers, Ander and Powell10 In septic shock, this is related to increased consumption of oxygen by the gut which is not balanced by an increase in regional delivery. This leads to increased extraction, and can result in tissue dysoxia.Reference Marx and Reinhart8 In the early phase of cardiogenic and hypovolaemic shock, there is a redistribution of blood away from splanchnic, renal and mesenteric beds, with preferential perfusion of the cerebral and coronary circulations. This also leads to increased extraction in the territory of the inferior caval vein.Reference Marx and Reinhart8, Reference Rivers, Ander and Powell10 In both situations, inferior caval venous saturation is reduced relatively early in the course of the disease, whereas flow of blood to the brain is initially maintained, leading to a delayed decrease in superior caval venous saturation.Reference Marx and Reinhart8, Reference Reinhart, Kuhn, Hartog and Bredle11 In patients in shock, therefore, superior caval venous saturation will typically overestimate mixed venous saturation, whereas inferior caval venous saturation may underestimate it. One can conclude that the interpretation of absolute values for central venous saturation instead of mixed venous saturation is at best unreliable, or may even be misleading, in children with shock.
All published data comparing central venous saturation to mixed venous saturation has arisen from animal models or adult studies, in a variety of clinical settings. These include health, septic shock, haemorrhagic shock, cardiac failure, myocardial infarction, and cardiac arrest. There is, however, no published data comparing superior or inferior caval venous saturation to mixed venous saturation in children, in particular in the post-operative cardiac population, when total and regional balance of oxygen is constantly fluctuating. In adults, there are a number of published series comparing superior caval venous saturation and true mixed venous saturation in a variety of pathological and haemodynamic states, which share the conclusion that, while absolute numbers differ substantially, depending on the site of sampling, the trends correlate well.Reference Marx and Reinhart8, Reference Rivers, Ander and Powell10, Reference Bloos and Reinhart12–Reference Reinhart and Bloos14
Goal directed therapy, targeting ideal mixed venous saturations, has been widely applied in various sub-populations of critically ill patients. For example, an improved outcome was demonstrated by targeting a superior caval venous saturation of greater than 70% when resuscitating those with severe sepsis and septic shock in the emergency department.Reference Rivers, Nguyen and Havstad15 Caution should be applied, however, when targeting absolute values in the presence of shock, because superior caval venous saturation may over-estimate mixed venous saturation in this setting, and a seemingly adequate value may introduce a false sense of security within the clinicians in charge of management. It does mean, however, that in patients with shock, a pathologically low superior caval venous saturation implies an even lower mixed venous saturation, which may require intervention. The difference in numeric value, therefore, does not necessarily detract from clinical usefulness, as long as clinicians take account of this when considering therapeutic strategies.Reference Rivers, Ander and Powell10, Reference Dueck, Klimek, Appenrodt, Weingand and Boerner13, Reference Reinhart, Rudolph, Bredle, Hannemann and Cain16
Venous saturations in the presence of complex congenital cardiac disease
The functionally univentricular circulation
In many cases of congenital cardiac disease the true mixed venous saturation is not directly measurable, due to mixing of systemic venous and pulmonary venous blood at multiple levels. This problem applies to infants with a functionally univentricular circulation, both pre-operatively and also following palliative procedures such as the Norwood operation or its modifications (Fig. 3), in whom systemic and pulmonary venous blood are mixed at the atrial level. Paradoxically, it is in these patients, where a reliable measure of mixed venous saturation of oxygen would be most useful, that it cannot be accurately assessed. Similarly, the true mixed venous saturation is unmeasurable in infants and children after a bi-directional cavo-pulmonary shunt, where superior caval venous blood flows directly into the pulmonary circulation, and inferior caval venous blood mixes with pulmonary venous blood in the effectively common atrial compartment. In older children with a Fontan circulation, all systemic venous blood flows directly to the pulmonary arteries, and there is no mixing chamber for the venous blood from the upper and lower parts of the body. In these complex circulations, the best available estimation of mixed venous saturation is the superior caval venous saturation.

Figure 3 Stylised representation of the functionally univentricular circulation, with complete mixing of systemic and pulmonary venous blood in a surgically created common atrium. Blood enters the pulmonary arteries via an extra-cardiac systemic-venous shunt, shown here as a modified Blalock-Taussig shunt, but a direct conduit from the right ventricle to the pulmonary arteries is also commonly used. Percentage saturations are approximate, assuming a ratio of pulmonary to systemic flow of 1 to 1.
Patients with extracardiac systemic-to-pulmonary arterial shunts
The interpretation of venous saturation in patients with an extracardiac systemic-to-pulmonary shunt is also very complex. In these patients, the total cardiac output is simultaneously distributed between the pulmonary and systemic circulations, and venous saturation reflects the balance between these circulations, in other words the ratio of pulmonary to systemic flow, commonly termed Qp:Qs. If total cardiac output remains constant, but the ratio of pulmonary to systemic flows increases, then venous saturation will fall as more oxygen is extracted from the tissues due to its reduced systemic delivery. Therefore, in interpreting a low venous saturation, it is necessary to consider both a low total cardiac output, due to pump failure and a low effective systemic cardiac output, with preserved total cardiac output, due to an increased ratio of pulmonary to systemic flow.
Patients with intracardiac right-to-left shunts
Intracardiac right-to-left shunting is present in a variety of congenital cardiac lesions, and results in the baseline arterial saturation being lower than in the biventricular septated heart. Thus, for a given consumption of oxygen by the tissues, the corresponding venous saturation will similarly be lower. The same is also true of those patients with pulmonary pathology and desaturated pulmonary venous blood. For example, in a patient with hypoplastic left heart syndrome after a Norwood-type procedure with arterial oxygen saturation of 75%, a central venous saturation of oxygen of 50% may be adequate, With this physiology, therefore, the absolute value of venous saturation is less useful than the arterio-venous difference in the saturation of oxygen, or the ratio of extraction of oxygen (Text Box 2).Reference Tibby and Murdoch6 Cardiac index has been shown to correlate much better with the inverse of the ratio of extraction of oxygen, known as the oxygen excess factor or Ω, than with mixed venous saturation of oxygen itself.Reference Buheitel, Scharf, Hofbeck and Singer17
The anaerobic threshold
If an increase in demand for, or consumption of, oxygen is not compensated by an adequate increase in delivery, then this will adversely impact upon mixed venous saturation. Similarly, the impact on overall balance of oxygen and mixed venous saturation of a fall in systemic delivery depends on whether or not there is a change in consumption. In general, significant and sustained increases in consumption, or falls in delivery, result first in a compensatory increase in extraction of oxygen, with a fall in mixed venous saturation. If this process continues without the balance being redressed, the so-called anaerobic threshold is exceeded, resulting in tissue hypoxia, anaerobic metabolism, and lactic acidosis. If this continues unabated, multi-organ failure develops, and death ensues.
The anaerobic threshold, and its detection by monitoring superior caval venous saturation, has been explored post-operatively in infants after surgery for complex cardiac disease, in particular after the Norwood operation. The utility of continuous monitoring of superior caval venous saturation alongside conventional acid-base balance has been extensively reported in young infants after the Norwood operation, arguably the procedure currently carrying the highest risk amongst those performed currently in neonates.Reference Hoffman, Ghanayem and Kampine18 In these infants, it has been demonstrated that the risk of anaerobic metabolism, defined as a standard base excess less than −4mEq/L or a change greater than −2mEq/L/hr, increased from 4.8% to 29% when superior caval venous saturation fell to 30% or less.Reference Marx and Reinhart8, Reference Bloos and Reinhart12, Reference Hoffman, Ghanayem and Kampine18 This anaerobic threshold would not have been detected on the basis of arterial saturation of oxygen or blood pressure. It is important to note that the threshold for anaerobic metabolism is likely to remain the same, at a central venous saturation of between 30–50%, irrespective of the baseline arterial saturation. In these patients with right-to-left shunting, therefore, there is less room for compensatory extraction before the onset of lactic acidosis, and ultimately cell death begins to occur.
Venous saturation and goal-directed therapy in high risk infants after surgery for congenital cardiac disease
A lower superior caval venous saturation, and wider arterio-venous difference, has been correlated with adverse outcome in terms of early survival, the need for extracorporeal life support, and neurodevelopmental morbidity, in infants with hypoplastic left heart syndrome.Reference Tweddell, Ghanayem and Mussatto19, Reference Hoffman, Mussatto and Brosig20 In this condition, a superior caval venous saturation of less than 55% was associated with the need for extra-corporeal membrane oxygenation, cardiopulmonary resuscitation, and early death. Targeting a higher threshold, between 55–60%, was suggested as optimal in achieving the lowest risk of mortality and complications.Reference Tweddell, Ghanayem and Mussatto19
The utility of intermittent, rather than continuous, monitoring by sampling blood rather than co-oximetry has also been examined after the Norwood operation. It was shown that intermittent monitoring enabled more accurate detection of abnormal ratios of pulmonary to systemic flow, and therefore could enable earlier adjustments in management in order to optimise this ratio, and hence improve post-operative outcomes.Reference Rossi, Sommer and Lotvin21
Over a decade ago, a number of parameters, including mixed venous saturation, were examined as potential predictors of major adverse events, defined as cardiac arrest, the need for emergency chest opening, or the development of multi-organ failure or death in children after cardiac surgery.Reference Duke, Butt, South and Karl22 Mixed venous saturation was measured from indwelling pulmonary arterial catheters. In the patients studied, blood pressure, lactate, and the period of cardiopulmonary bypass, but not mixed venous saturation, were independent predictors of major adverse events. The cohort examined, however, included a proportion of infants with intracardiac mixing, such that pulmonary arterial saturation would not accurately reflect mixed venous saturation. This analysis perhaps highlights the importance of the site of sampling, and intracardiac shunting on the predictive ability of the venous saturation. In addition, although this studyReference Duke, Butt, South and Karl22 does not state the number of major adverse events due to shunt occlusion, of relevance may be the observation that monitoring of central venous saturation is of little additional benefit to conventional monitoring in identifying thrombosis of shunts, and that it does not eliminate the need for cardiopulmonary resuscitation or extracorporeal life support due to acute occlusion of the shunt.Reference Tweddell, Ghanayem and Mussatto19
Conclusion
The most important factors in approaching the monitoring of mixed venous saturation in paediatric cardiac care relates to whether or not this value can be measured, and then, as to the appropriate site of sampling. In patients with a septated biventricular heart without shunting, the pulmonary artery is the most appropriate site, though the superior caval vein would be a reasonable surrogate, if trends, rather than absolute values, are observed. The presence of shunting, which can be right-to-left, left-to-right, intracardiac or extracardiac, or surgical anatomy in which the systemic veins are in direct continuity with pulmonary arteries, means that in many cases, a true mixed venous saturation cannot be measured. Paradoxically, it is precisely in these patients, typically after staged palliation in early infancy, that a marker of adequacy of systemic delivery of oxygen would be of greatest utility. While still somewhat controversial, there is evolving consensus that trends in central venous saturation may still be clinically useful in these patients. Interpretation of central venous saturation in patients with a functionally univentricular heart is complex, as this can represent both a reduced total cardiac output, or more commonly a maldistribution of cardiac output with increased pulmonary flow, and reduced systemic delivery of oxygen. Finally, in patients with a low baseline arterial saturation of oxygen, consideration of the arterio-venous difference, or the ratio of extraction of oxygen, can prove useful in addition to the absolute values of central venous saturation.





