Systemic-to-pulmonary shunts are implanted in children with cardiac malformations to ensure sufficient pulmonary flow and subsequent peripheral oxygenation. Complications after systemic-to-pulmonary shunt implantation, including shunt thrombosis and death, are unfortunately still common.Reference Li, Yow and Berezny 1 , Reference Cholette, Rubenstein and Alfieris 2 Acetyl salicylic acid is widely used to prevent shunt thrombosis after shunt implantation and appears to reduce the risk.Reference Li, Yow and Berezny 1
Acetyl salicylic acid is also used to treat and prevent thrombosis in a large number of other pathological conditions, including coronary artery disease and cerebral vascular disease. Acetyl salicylic acid blocks the action of cyclooxygenase, which leads to inhibition of platelet aggregation and also to a decreased inflammatory reaction.Reference Vane and Botting 3 However, the effect of acetyl salicylic acid on platelet aggregation is rarely monitored, even though it is well known that the response to acetyl salicylic acid varies among individuals. Adult studies report 5–51% prevalence of acetyl salicylic acid resistance.Reference Angiolillo, Fernandez-Ortiz and Bernardo 4 – Reference Valles, Santos and Aznar 6 In paediatric cardiac surgery, acetyl salicylic acid resistance was found in 14–43% of the patients,Reference Heistein, Scott and Zellers 7 , Reference Cholette, Mamikonian, Alfieris, Blumberg and Lerner 8 with cyanotic patients more likely to be resistant.Reference Heistein, Scott and Zellers 7
Impedance aggregometry is a new method for monitoring platelet aggregation.Reference Toth, Calatzis, Penz, Losconczy and Siess 9 The method has mainly been used to monitor anti-platelet therapy with acetyl salicylic acid and clopidogrel in adult patients with coronary artery disease.Reference Jambor, Weber and Gerhard 10 – Reference Velik-Salchner, Maier and Innerhofer 12 The primary aim of the present study was to determine the effects of acetyl salicylic acid medication on platelet aggregation in children with systemic-to-pulmonary shunts. A secondary aim was, because of the high incidence of thrombotic events in this patient group, to examine the prevalence of pre-existing haemostatic disturbances by analysing standard coagulation tests. For these purposes a prospective observational study was conducted in children with systemic-to-pulmonary shunts.
Material and methods
Patients and procedures
The study was approved by the Regional Medical Research Ethics Committee. Written informed consent was provided by all parents. A total of 14 patients with a median age of 12 days – ranging from 3 to 100 – and median weight of 3.5 kilograms – ranging from 2.2 to 4.6 – were prospectively included in a prospective observational study between 2007 and 2009. Patients with a known coagulation defect or severe renal or hepatic disorder were excluded from participation. Patient demographics and surgical procedures are presented in Table 1.
Table 1 Patient characteristics, diagnosis, procedures and acetyl salicylic acid dose.

ASA 1 = initial ASA dose; ASA 2 = adjusted ASA dose after 3–6 months treatment; AV = atrial-ventricular septal defect; BT = modified Blalock–Taussig shunt; C = central shunt; DO = double outlet right ventricle; F = female; HL = hypoplastic left heart syndrome; M = male; ND = Norwood procedure; PA = pulmonary atresia; S = Sano shunt
A Sano shunt was implanted in eight children, a modified Blalock–Taussig shunt in five, and a central shunt in one child. Of the children, seven underwent a Norwood procedure in addition to shunt implantation. All children were treated post-operatively with unfractioned heparin (Leo Pharma A/S, Ballerup, Denmark; 250 international units per kilogram daily). Once oral feeding was established – on post-operative days 1–3 – acetyl salicylic acid treatment was started at a dose of 3–5 milligrams per kilogram once daily.
Routine and coagulation analyses
Routine laboratory analyses were carried out at three time points: (1) before the primary shunt operation; (2) before the first acetyl salicylic acid dose (post-operative days 1–3); and (3) after 3–6 months of acetyl salicylic acid treatment – in conjunction with routine cardiac catheterisation. The analyses – serum-creatinine, blood urea nitrogen, aspartate amino transferase, alanine amino transferase, white blood cell count, platelet count, blood haemoglobin, and haematocrit – were carried out using standard clinical methods.
At the same time points the following coagulation analyses were also carried out to determine: activated thromboplastin time (institutional reference range for neonates less than 30 days: 31–55 seconds; more than 30 days: 30–42 seconds), prothrombin time (1.0–1.7 international normalised ratio), coagulation factor V plasma activity (day 1: 0.34–1.08 kilo international units per litre; day 5: 0.45–1.45 kilo international units per litre; day 90: 0.48–1.32 kilo international units per litre), plasma fibrinogen level (2.0–4.5 grams per litre), D-dimer level (0–0.5 milligram per litre), anti-thrombin level (less than 30 days: 0.48–1.08 kilo international units per litre; more than 30 days: 0.80–1.20 kilo international units per litre), protein C level (less than 30 days: 0.17–0.65 kilo international units per litre; more than 30 days: 0.37–0.81 kilo international units per litre), and protein S level (less than 30 days: 0.33–0.93 kilo international units per litre; more than 30 days: 0.70–1.50 kilo international units per litre) (Table 2). All samples were analysed by an accredited university hospital laboratory using equipment from Diagnostica Stago (Asniers, France).
Table 2 Routine laboratory variables and coagulation variables before and after shunt implantation and 3–6 months later – mean and standard deviation

ALAT = alanine amino transferase; APTT = activated prothrombin time; ASAT = aspartate amino transferase; g = gram; INR = international normalised ratio; kIU = kilo international units; L = litre; PK = prothrombin time; ukat = microkatal; WBC = white blood cell count;
**p < 0.01 versus pre-operative
***p < 0.001 versus preoperatively
Impedance aggregometry
Platelet aggregation and platelet count were analysed at five time points: (1) before the primary shunt operation; (2) before the first acetyl salicylic acid dose; (3) 5 hours after the first acetyl salicylic acid dose; (4) 24 hours after the first acetyl salicylic acid dose; and (5) after 3–6 months of acetyl salicylic acid treatment. The immediate response to acetyl salicylic acid treatment was calculated as the difference between measurement number 2 – before acetyl salicylic acid treatment – and measurement number 3 – 5 hours after acetyl salicylic acid treatment. In all other analyses the baseline is the pre-operative measurement before the primary shunt operation.
Blood for impedance aggregometry was drawn from the arterial line and collected in 4-millilitre lithium–heparin tubes (Greiner Bio-One, International AG, Kremsmünster, Austria). Resting time for the samples before analysis was 30 minutes. Platelet aggregation was analysed with impedance aggregometry (Multiplate®, Verum Diagnostica GmbH, Munich, Germany) in whole blood. Detailed descriptions of the method have been published previously.Reference Cholette, Mamikonian, Alfieris, Blumberg and Lerner 8 , Reference Toth, Calatzis, Penz, Losconczy and Siess 9 , Reference Paniccia, Antonucci and Maggini 11 In short, the Multiplate® device has five test cells for parallel testing, and each test cell has two independent sensor units to reduce systemic errors. Each unit consists of two silver-coated, highly conductive copper wires. Analysis is based on platelet adhesion, which results in aggregation onto the metal sensor wires in the test cell, resulting in increased electrical impedance between the wires. According to the manufacturer, platelet count above 100 × 109 per litre should yield valid results.
The measurements were taken in the test cell with 300 microlitres of pre-heated saline (37° Celsius) and 300 microlitres of heparin anti-coagulated whole blood. The sample was stirred (800 rotations per minute) during a 3-minute incubation time. Platelet aggregation was initiated after addition of arachidonic acid (ASPI-test, 0.5 millimolar, 20 microlitres), thrombin receptor-activating peptide (TRAP-test, 32 micromolar, 20 microlitres), and adenosine diphosphate (ADP-test, 6.5 micromolar, 20 microlitres), all supplied by the manufacturer. Impedance changes due to platelet aggregation were continuously measured for 6 minutes for each test cell. Platelet aggregation was measured in arbitrary units as the area under the aggregation curve.
Reference values with heparin tubes are as recommended by the manufacturer for ASPI-test (79–141 units), ADP-test (55–117 units), and TRAP-test (87–147 units). The therapeutic range of acetyl salicylic acid was defined as an ASPI-test result with heparin tubes less than 60 units according to the manufacturer's recommendation. For hirudin tubes the corresponding therapeutic range has been suggested to be less than 30 units.Reference von Pape, Dzijan-Horn, Bohner, Spannagl, Weisser and Calatzis 13
Statistical analyses
The results are presented as mean with standard deviation unless otherwise indicated. The paired Student t-test was used to compare post-operative measurements with pre-operative measurements. A p-value of less than 0.05 was considered statistically significant. Statistical analyses were conducted with SPSS 13.0 for Windows (SPSS Incorporation, Chicago, Illinois, United States of America). No sample size calculation was performed. The study was descriptive and longitudinal, and the patients served as their own controls. All eligible patients at our institution between 2007 and 2009 were included in the study.
Results
General
The first four measurements could be obtained for all children. Of the 14 children, 11 completed the total study time (3–6 months) with acetyl salicylic acid treatment. Of the three missing patients, one patient underwent acute surgery after 3 months (before sampling) because of shunt dysfunction, one patient was switched to dicumarol treatment after 2 weeks because of shunt dysfunction, and one patient's sample was missed after 3–6 months. The mean starting dose of acetyl salicylic acid was 4.9 plus or minus 0.6 milligram per kilogram – ranging from 4.0 to 5.7 – and the mean acetyl salicylic acid dose at the end of the study period after 3–6 months of treatment was 3.6 plus or minus 0.9 milligram per kilogram – ranging from 2.6 to 4.7. Eight children received perioperative transfusion of platelets. Median dose was 50 millilitres – ranging from 30 to 80 millilitres. Patient's characteristics and surgical procedures are given in Table 1.
Routine and coagulation analyses
Pre-operative
In all, seven children had fibrinogen levels and five children had anti-thrombin levels below the reference range, whereas seven children had D-dimer concentrations above the reference range. Mean concentrations are given in Table 2.
Post-operative
Platelet count, Factor V activity, D-dimer, anti-thrombin, and protein S concentrations were all lower post-operatively compared with the pre-operative measurements, whereas creatinine, blood urea nitrogen, aspartate amino transferase, and alanine amino transferase concentrations were higher post-operatively than pre-operatively (Table 2). Activated thromboplastin time was longer post-operatively because of heparin treatment. Post-operatively, 11 children had anti-thrombin, nine had fibrinogen, and seven had protein S levels below the reference level.
3–6 months after shunt implantation
Compared with the pre-operative measurements, creatinine levels were lower and anti-thrombin, protein C, and protein S concentrations were higher; no other variable was significantly different (Table 2).
Platelet count and impedance aggregometry
Platelet count
Mean pre-operative platelet count was 369 plus or minus 167 × 109 per litre (ranging from 139 to 709) pre-operatively, which fell to 200 plus or minus 97 × 109 per litre (ranging from 40 to 383) post-operatively (p is equal to 0.002). At 5 hours after the first acetyl salicylic acid dose, platelet count was 196 plus or minus 85 × 109 per litre (ranging from 96 to 379; p is equal to 0.002) compared with pre-operative count; 24 hours after acetyl salicylic acid dose, platelet count was 196 plus or minus 69 × 109 per litre (ranging from 100 to 332; p is equal to 0.002) versus preoperatively, and after 3–6 months the count was 392 plus or minus 126 × 109 per litre (ranging from 148 to 556; p is equal to 0.94) versus preoperatively.
ASPI-test (Fig 1a)
One child showed a pre-operative ASPI-test result (77 units) just below the lower normal limit – reference range from 79 to 141 units. Acetyl salicylic acid reduced the immediate salicylic acid-dependent platelet aggregation in all but one patient (from mean 86 plus or minus 21 to 35 plus or minus 13 units; p is less than 0.001). When compared with pre-operative levels, the first post-operative ASPI-test score did not differ significantly (p is equal to 0.13); however, the scores were significantly lower at all later time points (5 hours, 24 hours, and 3–6 months after surgery), Figure 1a. In all, 13 out of 14 patients (93%) were in the therapeutic range for acetyl salicylic acid treatment (ASPI-test lesser than 60 units) 5 hours after the first acetyl salicylic acid dose, 12 out of 14 (86%) after 24 hours, and 7 out of 11 (64%) after 3–6 months of acetyl salicylic acid treatment (Fig 2).
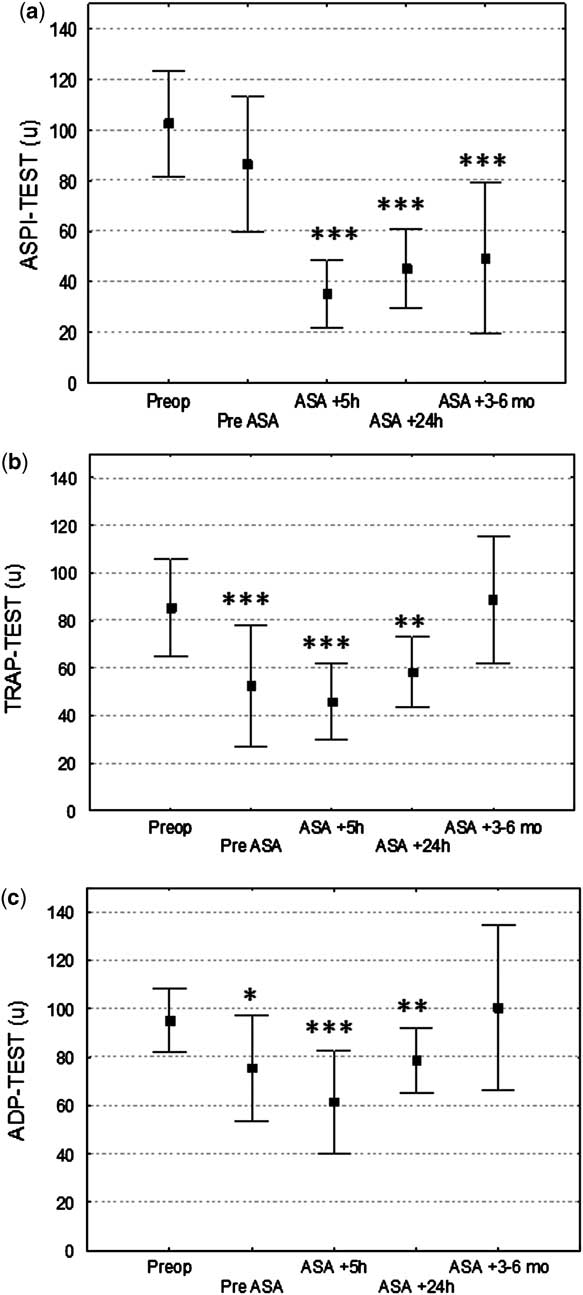
Figure 1 Impedance aggregometry with the arachidonic acid (ASPI)-test (a), the thrombin receptor-activating peptide (TRAP)-test (b), and the adenosine diphosphate (ADP)-test (c) before and after acetyl salicylic acid medication in children operated upon with systemic-to-pulmonary shunts. Mean plus or minus standard deviation. *p < 0.05 versus pre-operative, **p < 0.01 versus pre-operative, ***p < 0.001 versus pre-operative.

Figure 2 Percentage of patients within the therapeutic range for acetyl salicylic acid treatment (arachidonic acid (ASPI)-test lesser than 60 units).
TRAP-test (Fig 1b)
The pre-operative TRAP-test score was below the lower normal limit (87–147 units) in six children. There was no significant immediate effect of acetyl salicylic acid on the results of the TRAP-test (before acetyl salicylic acid treatment: 76 plus or minus 22 units; after acetyl salicylic acid treatment: 63 plus or minus 21 units, p is equal to 0.08). The mean TRAP-test result was significantly lower than the pre-operative result at the first three post-operative measurements but had returned to pre-operative levels at the measurements taken 3–6 months later.
ADP-test (Fig 1c)
All children had an ADP-test result within reference values (55–117 units). There was no significant immediate effect of acetyl salicylic acid on ADP-test results (before acetyl salicylic acid treatment: 52 plus or minus 25 units; after acetyl salicylic acid treatment: 56 plus or minus 16 units; p is equal to 0.39). The ADP-test result was significantly lower than the pre-operative score at the three post-operative measurements but had returned to pre-operative levels at the measurements taken 3–6 months later.
Discussion
The main findings of the present study are as follows: (1) acetyl salicylic acid reduces arachidonic acid-induced platelet aggregation in children with systemic-to-pulmonary shunts; (2) a substantial percentage of the patients are outside the previously suggested therapeutic ranges for acetyl salicylic acid; (3) low anti-thrombin levels are common both pre- and post-operatively in patients operated upon with systemic-to-pulmonary shunts.
Shunt thrombosis after systemic-to-pulmonary shunt implantation is common. Cholette et alReference Cholette, Rubenstein and Alfieris 2 found that, after initial palliative surgery with shunts in patients with single ventricle physiology, 28% of survivors had evidence of shunt thrombosis. In that study, elevated pre-operative C-reactive protein values appeared to be associated with increased thrombosis risk. Wells et alReference Wells, Yu, Batra, Monforte, Sintek and Starnes 14 demonstrated that most modified Blalock–Taussig shunts had developed stenosis by the time of takedown, and 21% had a greater than 50% obstruction, which may predispose to acute thrombosis. The same authors identified a shunt size less than 4 millimetres as a risk factor for high-grade stenosis. Because of the significantly increased risk for thrombotic events in children with systemic-to-pulmonary shunts, treatment with acetyl salicylic acid is generally recommended.Reference Monagle, Chalmers and Chan 15 This recommendation is based on a large observational multicentric study conducted by Li et alReference Li, Yow and Berezny 1 in which reduced prevalence of shunt thrombosis and improved survival were observed if acetyl salicylic acid was used, especially after the Norwood procedure.
However, the effect of acetyl salicylic acid is rarely monitored, despite evidence that a significant percentage of children have an impaired response to acetyl salicylic acid.Reference Heistein, Scott and Zellers 7 , Reference Cholette, Mamikonian, Alfieris, Blumberg and Lerner 8 , Reference Israels and Michelson 16 An overall acetyl salicylic acid resistance prevalence of 26% in paediatric cardiac surgery patients has previously been reported with increased prevalence in children with cyanotic heart disease (39.5%).Reference Heistein, Scott and Zellers 7 There is still no clear explanation why cyanotic patients are more likely to be acetyl salicylic acid resistant. Increased platelet turnover, enhanced degranulation, and platelet hyper-reactivity to other agonists such as adenosine diphosphate have been proposed as contributing factors. Reference Gross, Keefer and Liebman 17 , Reference Waldman, Czapek, Paul, Schwartz, Levin and Schindler 18 Platelets from children with cyanotic heart disease have been reported to have an increased initial rate of aggregation and greater maximum aggregation.Reference Goldschmidt and Sorland 19
In the present study, acetyl salicylic acid reduced the immediate arachidonic acid-induced platelet aggregation in all but one patient. In contrast, acetyl salicylic acid did not have any significant effect on adenosine diphosphate and thrombin-activating peptide-dependent aggregation. This indicates that the reduction in acetyl salicylic acid-dependent aggregation is specific and not caused by a general post-operative reduction in platelet count or platelet function. The response varied considerably with acetyl salicylic acid-dependent platelet inhibition ranging from 20% to 79%, which supports the concept that acetyl salicylic acid response needs to be monitored. The variation in response is in accordance with that seen in previous studies.Reference Heistein, Scott and Zellers 7 , Reference Cholette, Mamikonian, Alfieris, Blumberg and Lerner 8 , Reference Israels and Michelson 16 It is, however, difficult to compare the immediate platelet inhibition in the present study with previous observations as the pre-acetyl salicylic acid values were influenced by the surgical procedure. It is obvious from Figure 1 that the surgical procedure suppressed platelet aggregation with statistically significant reductions in adenosine diphosphate and thrombin-activating peptide-initiated aggregation, whereas acetyl salicylic acid-dependent aggregation tended to be impaired (p is equal to 0.13). Thus, the platelet inhibition at 5 and 24 hours after acetyl salicylic acid treatment is influenced by both the surgical procedure and the acetyl salicylic acid. This may also explain why a larger proportion of patients were within the therapeutic range at the earlier measurements compared with 3–6 months later, when the effect of surgery on platelet aggregation was no longer present. Thus, our results indicate that the current recommended dose of acetyl salicylic acid (1–5 milligrams per kilogram) may be insufficient in some patients after the early post-operative period and that either a higher dose of acetyl salicylic acid or a combination of platelet inhibitors is necessary. It may also be speculated that monitoring the effect of platelet inhibition can be used to tailor individual doses and thereby ensure sufficient platelet inhibition in all patients.
An alternative explanation for the large proportion of patients outside the therapeutic interval 3–6 months after implantation is lack of compliance. This is a less likely explanation as the parents of children with shunts are well informed about the risk for shunt thrombosis and the importance of acetyl salicylic acid treatment. Hence, suboptimal dosing and variations in response to acetyl salicylic acid are more plausible explanations.
The majority of standard coagulation tests before surgery were within the normal range. Post-operative measurements were, as expected, influenced by the surgical procedure. However, a substantial proportion of the patients – 5 out of 14 before surgery and 11 out of 14 after surgery – had anti-thrombin levels below the lower normal limit. This interesting finding may also relate to the development of shunt thrombosis. The low levels of anti-thrombin may be deleterious in the early post-operative phase when heparin is used for anti-coagulation as heparin requires anti-thrombin for its action. Our finding confirms previous studies in paediatric cardiac surgery in which low post-operative levels of anti-thrombin and other anti-thrombotic factors have been reported.Reference Heying, Oeveren and Wilhelm 20 – Reference Chan, Leaker and Burrows 22 Furthermore, seven patients had low protein S levels post-operatively, which also may contribute to an increased risk for shunt thrombosis.
In the present study, platelet aggregability was monitored with multi-electrode impedance aggregometry. Impedance aggregometry has been shown to correlate with other established platelet aggregation tests.Reference Paniccia, Antonucci and Maggini 11 , Reference Velik-Salchner, Maier and Innerhofer 12 , Reference Siller-Matula, Gouya, Wolzt and Jilma 23 However, there are two important issues to discuss. First, the reference ranges used in the present study are from studies assessing adult patients as no study has established reference values in children using heparin as an anti-coagulant. Halimeh et al recently published impedance aggregometry results from healthy infants and children using hirudin tubes and reported a significant variation between different age groups for ASPI and TRAP-tests but not for ADP-test.Reference Halimeh, Angelis and Sander 24 These results were supported by the pre-operative analyses in the present study in which a considerable percentage of the children had aggregate values below the adult reference range with ASPI and TRAP-tests, whereas the ADP-test was within the adult reference range for all children. However, if impedance aggregometry is to be used clinically in children, reference values for different age groups and for different anti-coagulants need to be established. Second, the therapeutic range for acetyl salicylic acid with impedance aggregometry is not well defined. The developer of the test recommends that acetyl salicylic acid-treated patients have an ASPI-test score below 60 units with heparin tubes, and this range was used in the present study. Others have suggested the lower normal limit for heparin tubes to be 51 units.Reference Rahe-Meyer, Winterhalter and Hartmann 25 If this lower limit was used to define the therapeutic range instead of the 60 units, 86%, 64%, and 64% – 5 hours, 24 hours, and 3–6 months after shunt implantation – of the patients would have been in the therapeutic range – compared with 93%, 86%, and 64% with the 60-unit limit. Another option would be to use the relative reduction in impedance aggregometry to identify patients within the therapeutic range. If a greater than 50% reduction in acetyl salicylic acid-dependent aggregability compared with the pre-operative value was considered sufficient, this would lead to 93%, 57%, and 64% – 5 hours, 24 hours, and 3–6 months after shunt implantation – of patients being within the therapeutic range. Independent of definition, the study shows that a large percentage of the patients were outside the therapeutic range, especially after 3–6 months.
The main limitation of this study is the sample size. The study should be regarded as a pilot investigation and the results should be interpreted with caution. Larger multicentric studies are warranted to further determine the value of monitoring acetyl salicylic acid response after systemic-to-pulmonary shunt implantation in children with congenital heart disease. Another limitation is that impedance aggregometry data are lacking at the time of complications in the two patients who developed shunt thrombosis during follow-up. It is unknown whether platelet inhibition was sufficient at the time of thrombosis. Platelet inhibition 5 and 24 hours after the first acetyl salicylic acid dose in these two patients did not differ in comparison with that in the remaining patients.
In conclusion, acetyl salicylic acid reduces platelet aggregation after shunt implantation in paediatric patients; however, a considerable percentage of the children are outside the therapeutic range. Monitoring of platelet aggregation has the potential to improve anti-platelet treatment after shunt implantation by identifying children with impaired acetyl salicylic acid response.
Acknowledgements
The authors thank Anna-Lena Janson and Lena Larsson for excellent technical assistance. The study was supported by the Västra Götaland (ALF/LUA grant), Sahlgrenska University Hospital, and The Swedish Heart and Lung Foundation. The study sponsors had no influence on the analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.






