Cor triatriatum is the rarest of all congenital cardiac diseases, accounting for 0.1–0.4% of congenital heart diseases. Reference Richardson, Doty, Siewers and Zuberbuhler1 It is an anomaly that has a fenestrated fibromuscular diaphragm covered by endocardium. It separates the upper common pulmonary venous chamber from the lower true left atrial cavity. The spectrum of disease correlates with the degree of obstruction caused by the membrane. Patients with significant obstruction are likely to present in infancy with symptoms resulting from pulmonary congestion and pulmonary arterial hypertension. Common presentations include failure to thrive, dyspnoea, cyanosis, or even shock. Reference Burger2 Although cor triatriatum can be isolated cases, it is associated with other congenital cardiovascular anomalies. Reference Yaroglu Kazanci, Emani and McElhinney3 Atrial septal defect is a common defect associated with cor triatriatum. Pulmonary venous anomalies and the persistent left superior caval vein are also often associated with cor triatriatum. Reference Kumar, Singh, Mishra and Thingnam4 Cor triatriatum is often associated with biventricular heart disease, however, we sometimes recognise that cor triatriatum is associated with univentricular heart disease such as hypoplastic left heart syndrome. Reference Naito, Harada and Uchita5–Reference Humpl, Reineker, Manlhiot, Dipchand, Coles and McCrindle7 The univentricular disease requires palliation to regulate pulmonary blood flow before or concurrently the operation of cor triatriatum. It is unknown whether the palliation procedure influences the incidence of pulmonary venous stenosis in patients with cor triatriatum. In addition, a previous study reported that the prognosis of all cor triatriatum includes biventricular and univentricular prognoses. Reference Saxena, Burkhart, Schaff, Daly, Joyce and Dearani8 No study compares the prognosis associated with a univentricular physiology or biventricular physiology. Therefore, we compared the operation period, survival rate, and incidence of pulmonary venous stenosis with univentricular and biventricular in our institution.
Materials and methods
Ethics statement
Kobe Children’s Hospital Institutional Review Board approved this retrospective study (Protocol no. 2-57), and individual written informed consent was waived.
Patients
The 24 patients diagnosed with cor triatriatum who underwent surgical repair at our institution between March, 2000 and July, 2020 were included in this study. These patients were classified into two groups according to whether they had univentricular physiology: univentricular group (n = 13) and biventricular group (n = 11). Patients in the univentricular group, except one patient who had a primary Norwood procedure, underwent palliative surgery to adjust pulmonary blood flow, such as a Blalock–Taussig shunt or pulmonary artery banding. In our cases, there was no pulmonary venous obstruction due to the pulmonary vein itself before cor triatriatum resection. Pulmonary vein obstruction symptoms arose due to narrowing atrial communication or narrowing between the atrium and accessory chamber.
We used the modified classification of Lucas for the morphologic classification of cor triatiatum Reference Herlong, Jaggers and Ungerleider9 (Table 1).
Table 1. Classification of cor triatriatum according to Lucas

Reprint from Herlong et al. Reference Saxena, Burkhart, Schaff, Daly, Joyce and Dearani8
Statistics
This study was a retrospective case-series study conducted at a single institution. Medical history, operative records, echocardiogram and catheterisation data, and outpatient clinical records were reviewed. Comparisons between groups were performed using the Mann–Whitney U test for data that were not normally distributed. A p-value of <0.05 was considered statistically significant. Survival analysis was performed according to the Kaplan–Meier method. Data analysis was performed using R version 3.5.1 (R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/).
Results
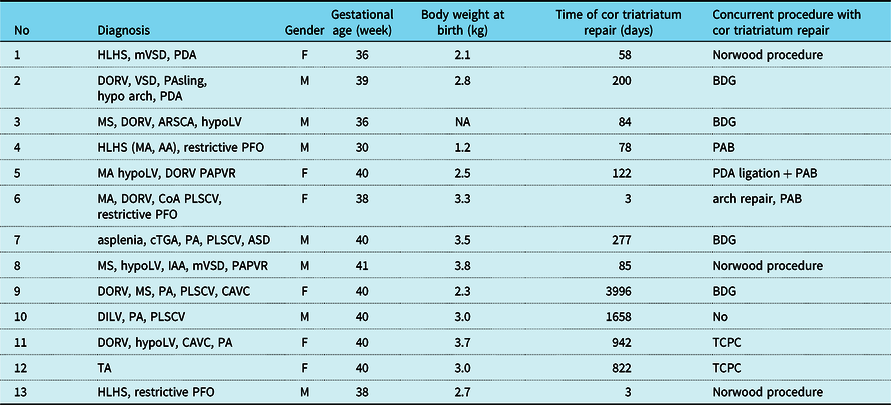
Tables 2 and 3 show the clinical characteristics of each group. The median gestational age was 39.5 (25th–75th percentiles: 37.3–40.0) weeks, birth weight was 3.1 (2.9–3.2) kg, and the median age at the operation of cor triatriatum was 158 (113–290) days in the biventricular group. The median gestational age was 40 (38.0–40.0) weeks, birth weight was 2.9 (2.5–3.4) kg, and the median age at the operation of cor triatriatum 122 (78–822) days in the univentricular group. Gestational age, birth body weight, and age at operation of cor triatriatum were not significantly different between the groups (Tables 2 and 3).
Table 2. Clinical characteristics of univentricular patients

ARSCA = aberrant right subclavian artery; ASD = atrial septal defect; BDG = bidirectional Glenn anastomosis; CAVC = complete atrioventricular canal; CoA = aortic coarctation; cTGA = corrected transposition of great arteries; DILV = double-inlet left ventricle; DORV = double-outlet right ventricle; HLHS = hypoplastic left heart syndrome; IAA = interrupted aortic arch; LV = left ventricular; MA = mitral valve atresia; MS = mitral valve stenosis; mVSD = muscular ventricular septal defect; PA = pulmonary atresia; PAB = pulmonary artery banding; PAPVR = partial anomalous pulmonary venous return; PA sling = pulmonary artery sling; PDA = patent ductus arteriosus; PFO = patent foramen ovale; PLSCV = patent left superior caval vein; TA = tricuspid atresia; TCPC = total cavopulmonary bypass.
Table 3. Clinical characteristics of biventricular patients
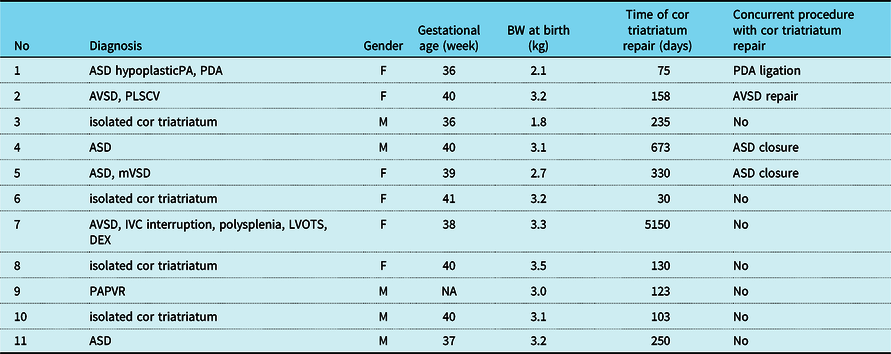
ASD = atrial septal defect; AVSD = atrioventricular septal defect; DEX = dextrocardia; IVC = inferior caval vein; LVOTS = left ventricular outflow tract stenosis; mVSD = muscular ventricular septal defect; PA = pulmonary artery; PAPVR = partial anomalous pulmonary venous return; PDA = patent ductus arteriosus; PLSCV = patent left superior caval vein.
Morphology
Twelve patients (50%) were type IA, six patients (25%) were IB1, one patient (4%) was type IB2, four patients (17%) had type IIA, and one patient (4%) had type IIIA2 in all patients.
Follow-up and mid-term outcomes
The median follow-up interval after cor triatriatum repair was 4.5 years (range: 0–8.8 years).
All patients experienced resection of the cor triatriatum membrane with surgery. Concurrent surgical procedures of cor triatriatum repair were performed in 18 patients (75%). In the univentricular group, four patients underwent the bidirectional Glenn procedure, three patients underwent with the Norwood procedure, two patients underwent with total cavopulmonary bypass, one patient underwent with the arch repair and main pulmonary artery banding, one patient underwent with pulmonary artery banding, one patient underwent ductus arteriosus ligation, and pulmonary artery banding. In the biventricular group, three patients had atrial septal defect closure, one had atrioventricular septal defect repair, one patient had PDA ligation, and one patient had partial anomalous pulmonary venous return repair. The survival rate of 5 years after cor triatriatum resection was 100% in the biventricular group and 82.1% in the univentricular group (Fig 1). There was no statistical difference. There was one patient who died 30 days after cor triatriatum repair and the Norwood procedure. His diagnose was hypoplastic left heart syndrome who required mechanical circulatory support for a cardiac failure during the peri-operative period and could not subsequently be weaned off. There was one late death, 1.8 years after cor triatriatum resection. His diagnose was double-outlet right ventricle, mitral valve atresia, coarctation with a univentricular circulation, and died from cardiac failure after the Norwood procedure.

Figure 1. Kaplan–Meier curves demonstrating survival rate over years after cor triatiatum resection.
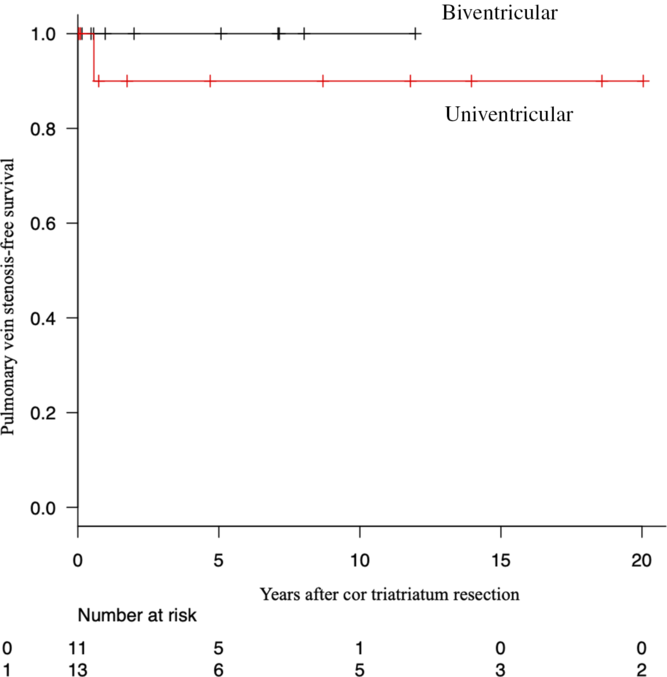
No patient underwent reintervention for recurrent cor triatriatum. The free rate for pulmonary stenosis of 5 years after surgery was 100% in the biventricular group and 90.0% in the univentricular group (Fig 2). There was no statistical difference. There was one pulmonary venous stenosis, 4 months after cor triatriatum resection, and the Norwood procedure in a patient with hypoplastic left heart syndrome. When we performed cardiac catheterisation for bidirectional Glenn procedure, the left pulmonary artery angiogram showed no left lower pulmonary vein flow. After cor triatriatum resection and the Norwood procedure, he required reintubation due to left atelectasis and phrenic nerve palsy.

Figure 2. Kaplan–Meier curves demonstrating pulmonary vein stenosis-free survival rate over years after cor triatiatum resection.
In this case, although the left lower pulmonary vein was obstruction, we could not diagnose it with ultrasound before the catheter exam and collect left lower pulmonary vein catheter data. Catheter results showed that central venous pressure was 9 mmHg, right ventricular pressure was 67 mmHg, mean left upper pulmonary vein pressure 11 mmHg, and mean left pulmonary artery pressure was 23 mmHg.
Discussion
The genetics of the cor triatriatum is not completely understood. Although multiple hypotheses are proposed, the most generally accepted hypothesis, as proposed by Van Praagh et al in 1969.
The incomplete insert of all the pulmonary veins into the left atrium causes arising in the persistence of a fibromuscular membrane between the common chamber draining pulmonary veins and the left atrium. Reference Kumar, Singh, Mishra and Thingnam4,Reference Van Praagh and Corsini10
This study has shown that cor triatriatum is associated with univentricular disease as well as biventricular disease, as seen in our institution. Although simple atrial septal defect and patent left superior caval vein are major complications associated with cor triatriatum, we should recognise that cor triatriatum may have many anatomic variants.
We diagnose cor triatriatum in various age and symptoms, and symptoms are dependent on the grade of pulmonary venous obstruction. Reference Humpl, Reineker, Manlhiot, Dipchand, Coles and McCrindle7
Cor triatriatum remains without symptoms until adult in some cases. If the patient were diagnosed cor triatriatum incidentally with no symptoms, we should check the membrane is not restrictive at regular follow-up. Reference Alphonso, Nørgaard, Newcomb, d’Udekem, Brizard and Cochrane6
This study showed that the median age at the operation of cor triatriatum was not different between the groups.
Palliative surgery to regulate pulmonary blood flow before the operation of cor triatriatum does not significantly influence symptoms due to cor triatriatum. Although there is no difference in survival rate, in the univentricular group, two patients died. Both patients underwent the Norwood procedure. It seems that cor triatriatum does not influence mortality directly after surgery.
However, it has a broad spectrum of presentations; infants can present with shock, pulmonary oedema, respiratory failure, and pulmonary hypertension in the most severe forms of this condition. Reference Burger2,Reference Gheissari, Malm, Bowman and Bierman11
It might raise mortality if the pre-surgery condition is an unwell such as shock even if isolated cor triatriatum. Most reported deaths occurred in children who presented in a critically ill condition, those with complex associated anomalies, or after a misdiagnosis. Reference Alphonso, Nørgaard, Newcomb, d’Udekem, Brizard and Cochrane6
Pulmonary vein stenosis was present in 4% of patients (n = 1) after cor triatriatum repair in our study; however, there is no recurrence of the left atrial membrane in patients.
Diagnosis is usually established by echocardiography; in our case, all patients were diagnosed by echocardiography. Reference Thakrar, Shapiro, Jassal, Neilan, King and Abbara12,Reference Hamdan, Mirochnik, Celermajer, Nassar and Iserin13
However, cor triatriatum has various patterns; contrast CT is more useful for recognising the detailed structure of cor triatriatum and underlying disease in complex heart disease patients. The standard treatment for cor triatriatum is surgery with excision of the membrane. We performed surgery to repair in all patients in our case.
Recently, successful percutaneous balloon dilatation of cor triatriatum has also been reported. There are no guidelines that are appropriate for percutaneous or surgical treatment. Reference Huang, Lee, Lin, Tseng and Hsieh14,Reference Schiller, Burns, Sinha and Cummings15
It seems that percutaneous balloon dilatation is a more reasonable treatment modality for isolated cor triatriatum; thus, avoiding thoracotomy, use of extracorporeal circulation, and cardioplegic arrest of the heart in these patients. It is associated with minimal mortality and morbidity. Reference Li, Koolbergen, Bouma, Hazekamp, de Mol and de Winter16
However, long-term data on morbidity and mortality using the percutaneous approach are unknown.
The results showed that surgical correction offers good early and mid-term outcomes for both cor triatriatum with biventricular and univentricular physiologies. The age at operation of cor triatriatum was not statistically different between the groups.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.