Vascular rings are congenital anomalies of the aortic arch that can lead to variable degrees of respiratory or feeding problems, through the formation of a complete or partial ring compressing the trachea and the oesophagus.Reference Kir, Saylam and Karadas 1 – Reference Backer, Mavroudis and Rigsby 5 Aberrant right subclavian artery with a left aortic arch, also known as arteria lusoria, is the most common type of vascular ring. It occurs in about 0.7–2% of the general population, and in a cadaveric study was found in 2.2%.Reference Edwards 6 – Reference Natsis, Didagelos and Gkiouliava 8 Most patients with isolated aberrant right subclavian artery are found incidentally. Respiratory distress, a history of recurrent pulmonary infections, apneic spells, and feeding problems are conventional indications for surgical intervention in patients with aberrant right subclavian artery.Reference Bull and Denck 2 – Reference Backer, Mavroudis and Rigsby 5 The development of innovative echocardiographic instruments has greatly improved the diagnostic accuracy of cardiovascular disorders. Echocardiography is an easy, non-radioactive, and non-invasive method that is now widely used for screening CHD in all age groups, particularly fetuses and infants.Reference Harcke and Walter 9 – Reference Rembouskos, Passamonti and De Robertis 16 However, literature-based systematic studies with a large cohort size regarding the use of echocardiographic screening to evaluate full-term neonates for detection of an aberrant right subclavian artery are sparse. The lack of existing data on this condition is an issue for physicians who follow up infants with an aberrant right subclavian artery, and remains a cause of considerable parental anxiety, as it is not always clear whether early surgery is warranted during infancy if the patient is asymptomatic. Thus, there is a need to prospectively assess such infants to determine whether passive follow-up or early surgical intervention is required. The purpose of this prospective study was to evaluate the incidence and clinical manifestations in full-term infants with an isolated aberrant right subclavian artery detected by echocardiographic screening during infancy and during mid-term follow-up. This study was presented at the 7th World Congress of Pediatric Cardiology and Cardiac Surgery in 2017 and published as a poster abstract.
Materials and methods
Patients
A total of 1737 unbiased consecutive full-term newborns (900 male and 837 female) with gestational ages ranging from 37 to 41+6 weeks received neonatal echocardiographic screening in a tertiary referral centre between January 2014 and March 2016 after written informed consent was obtained from their parents. The mean gestational age was 38.7 ± 1.5 weeks, and the mean birth weight was 3015 ± 643 g. Among these 1737 full-term neonates, a total of 35 infants were enrolled into the study: 20 age- and gender-matched normal infants and 15 infants with an isolated aberrant right subclavian artery. Their demographic data, perinatal history, and clinical manifestations were recorded and analysed during infancy, at birth, and at 3–5, 6–8, and 9–12 months. The exclusion criteria included neonates with other CHD, with the exception of patent ductus arteriosus, patent foramen ovale, and physiological valvular regurgitation. Also excluded were those with other systemic diseases, such as congenital central nervous system malformation, gastrointestinal, or genitourinary anomalies. Barium oesophagram was performed if aberrant right subclavian artery-related gastrointestinal symptoms were noted, such as dysphagia or feeding difficulty resulting in poor weight gain.
The aberrant right subclavian artery-related clinical characteristics and follow-up growth rates during infancy, including weight- and length-for-age percentiles, developmental milestones, gastrointestinal problems, and respiratory symptoms, were recorded and analysed. Their developmental milestones were assessed using formal milestone checklists in the Children’s Health Booklet, Health Promotion Administration, Ministry of Health and Welfare, Taiwan. Gastrointestinal problems and respiratory symptoms were documented following a parental questionnaire. Developmental delay was defined when an infant had not reached a developmental milestone, such as gross motor skills, fine motor skills, communication and language skills, and social skills, around the normally expected age. Developmental delay were divided into mild, moderate, and severe when delayed in one or more areas of developmental milestones at 1–3 months, 3–6 months, and more than 6 months, respectively. Aberrant right subclavian artery-related gastrointestinal and respiratory symptoms including feeding difficulty, gastro-oesophageal regurgitation, stridor or wheezing, frequent cyanosis of the lips, episodes of upper airway tract infection, and episodes of lower airway tract infection during the study period were divided into mild, moderate, and severe according to their severity, frequency, and the patient’s medical history. The severity levels of clinical manifestations were classified as mild, moderate, or severe, depending on how frequently the clinical manifestations occurred, and how frequently medical treatment was required. A mild degree was defined as aberrant right subclavian artery-related clinical manifestations occurring seldom, the frequency of infant regurgitation was once to four times a day, and the patient did not require medical treatment. A moderate degree was defined as the clinical manifestations occurring sometimes, when the frequency of infant regurgitation was more than four times a day, and the patient required conservative measures such as upright positioning after feeding, elevating the head of the bed, prone positioning, and providing small, frequent feeds thickened with cereal. A severe degree was defined as the clinical manifestations occurring often, when the frequency of infant regurgitation became a problem, and required pharmacologic interventions. Feeding difficulty was defined as a lengthy feeding time of more than 30 minutes. The length- and weight-for-age percentiles were based on the World Health Organization Child Growth Standards.
Echocardiographic study and diagnosis of aberrant right subclavian artery
Echocardiographic studies were performed using standard techniques according to the recommendations of the Committee on M-mode Standardization of the American Society of Echocardiography. All of the 1737 neonates between 2 and 4 days of age prospectively received a complete transthoracic echocardiography, including M-mode, two-dimensional, and colour Doppler flow mapping, using a Philips iE33 ultrasound system (Philips, Andover, Massachusetts, United States of America), in order to assess the aortic arch anomalies and coexisting CHD from different views. All echocardiographic studies were performed by two experienced paediatric cardiologists: S.-L. Jan and M.-C. Lin, who both serve as senior attending paediatric cardiologists at Taichung Veterans General Hospital Children’s Medical Center with over 10 years’ experience of echocardiographic practice. Normally, colour Doppler flow mapping shows that the left aortic arch branches into three blood vessels, including the brachiocephalic artery, left common carotid artery, and left subclavian artery, as in the standard long-axis suprasternal view (Fig 1a). Then after rotating the transducer counter-clockwise and tracing the brachiocephalic artery distally, the normal bifurcation of the right common carotid artery and right subclavian artery can be visually identified through its “fork” shape (Fig 1b). In patients with left aortic arch and aberrant right subclavian artery, echocardiographic visualisation of a non-branching first vessel originating from the left aortic arch that continued as the right common carotid artery served as a diagnostic clue for aberrant right subclavian artery (Fig 1c). Two separate arteries towards the right side, with the right common carotid artery and aberrant right subclavian artery arising from the posterior part of the aortic arch, can be visualised in a modified short-axis suprasternal view (Fig 1d). The same manipulative technique was performed in the opposite direction for the diagnosis of right aortic arch with aberrant left subclavian artery.
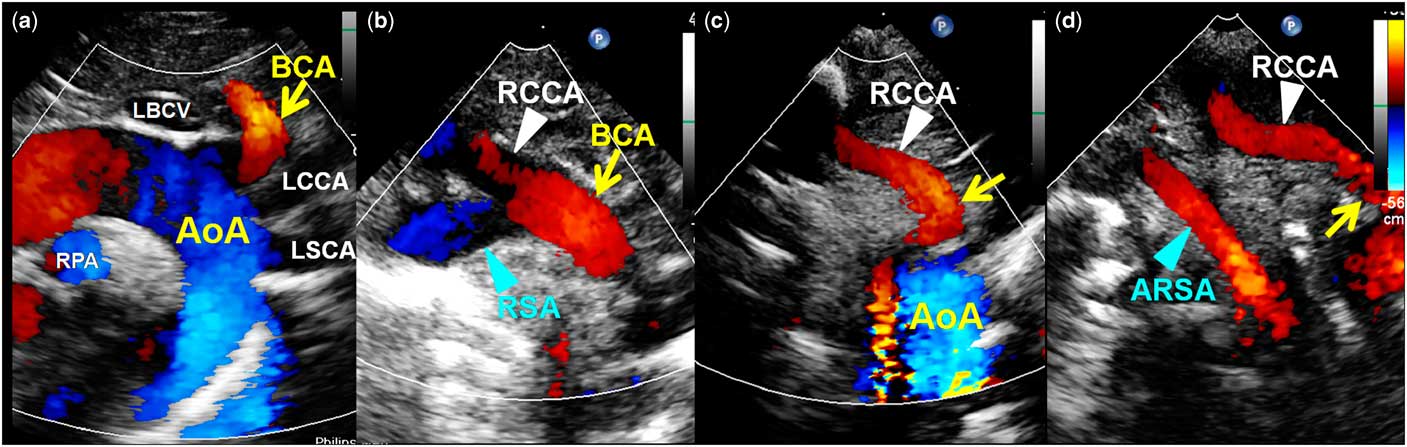
Figure 1 Comparison of colour Doppler aspect of infants with normal and aberrant right subclavian artery (RSA). The aortic arch (AoA) normally is left-sided and branches into three blood vessels ( a ). The first vessel of the AoA (brachiocephalic artery (BCA)) branches into right common carotid artery (RCCA) and RSA ( b ). A non-branching first vessel originating from the AoA (arrow) that continues as the RCCA ( c ), and two separate arteries towards the right side, RCCA and aberrant RSA (ARSA), can be visualised in patients with ARSA ( d ). LBCV=left brachiocephalic vein; LSCA=left subclavian artery; RPA=right pulmonary artery.
Statistical analysis
Results of the clinical symptoms were independently classified as asymptomatic, mild, moderate, or severe based upon the clinical presentations. Data are presented as mean ± standard deviation (range) or case numbers (percentage). The continuous variables for both groups were compared using the Mann–Whiney U test and the Wilcoxon rank-sum test or χ2 test. All tests of hypothesis were two-tailed. The level of significance was defined as values of p <0.05. Statistical tests were performed using the SPSS Statistics for Windows, version 22.0 (International Business Machines Corporation, Armonk, New York, United States of America).
Ethics approval
The experimental protocol was approved by the Taichung Veterans General Hospital Human Subjects Institutional Review Board (IRB TCVGH No.CF16042B).
Results
Among the 1737 full-term infants (male/female ratio of 1.075) who received neonatal echocardiographic screening, a left aortic arch and aberrant right subclavian artery was diagnosed in 16 (0.92%) cases and none of them had a right aortic arch with aberrant left subclavian artery. Aberrant right subclavian artery was an isolated echocardiographic finding in 15 (0.86%) infants. The remaining case experienced additional CHD, a muscular ventricular septal defect, and was thus excluded from this study. Among the 15 neonates with isolated aberrant right subclavian artery, there were more female than male infants (six male and nine female infants, with a male/female ratio of 0.667), but there was no statistically significant difference in gender ratio compared with that of the general population (p = 0.355). The 15 infants with isolated aberrant right subclavian artery (group I) were compared with 20 normal neonates without aberrant right subclavian artery (group II). Their demographic and perinatal data are shown in Table 1. There were no significant differences in maternal age, gestational age, or para gravity between the two groups. Infants with aberrant right subclavian artery had a smaller birth size (birth length- and weight-for-age percentiles, p = 0.006 and 0.045, respectively) and also a higher incidence of being small for gestational age (33.3 versus 0%, p = 0.023) than did the normal infants without aberrant right subclavian artery (Table 1). During the study period, a patient with aberrant right subclavian artery was lost to follow-up. The mean follow-up periods were 11.6 ± 1.8 months and 11.9 ± 1.0 in infants with or without aberrant right subclavian artery, respectively. The clinical characteristics and follow-up of body weight and length growth rates during the infant period were compared between the two groups (Table 2). There were no statistically significant differences in clinical manifestations, including developmental history, as well as gastrointestinal and respiratory symptoms. Neonates with aberrant right subclavian artery had higher incidences of mild developmental delay and mild feeding difficulty than those of normal neonates (21 versus 0%, p = 0.061; 36 versus 20%, p = 0.264, respectively), although not of statistical significance. The growth rates of body length- and weight-for-age percentiles during the infant period were not significantly different between the two groups (Fig 2). Because none of the enrolled cases displayed significant aberrant right subclavian artery-related gastrointestinal symptoms, such as dysphagia or moderate to severe feeding difficulty, none received an oesophagram during this study.

Figure 2 The length and weight percentiles of infants with isolated aberrant right subclavian artery ( a and b ) and normal infants ( c and d ) categorised by different ages. Differences of length- and weight-for-age percentiles between the two groups in the same age period and the p-values are shown at the bottom of the figure. BBL=birth length; BBW=birth weight; length-1 and weight-1=3–5 months of age; length-2 and weight-2=6–8 months of age; length-3 and weight-3=9–12 months of age.
Table 1 The demographic and perinatal data.

ASA=aberrant subclavian artery; LGA=large for gestational age; p%=percentile; SGA=small for gestational age
Data are presented as mean ± standard deviation (range)
*In the p value column, the comparisons were performed between the groups by the Mann–Whiney U test or χ2 test
Table 2 The clinical characteristics and follow-up of growth rates during infancy.

BBL=birth body length; BBW=birth body weight; LRTI=lower respiratory tract infection; p%=percentile; URTI=upper respiratory tract infection; △=difference between two values of percentile
Data are presented as mean ± standard deviation (range) or case numbers (percentage)
*In the p value column, the comparisons were performed between the groups by the Mann–Whiney U test or χ2 test
Discussion
Aberrant subclavian artery is one of the most common arch anomalies. Approximately 0.7–2% of the general population have a left aortic arch with aberrant right subclavian artery, whereas 0.04–0.4% have a right aortic arch with aberrant left subclavian artery.Reference Edwards 6 – Reference Natsis, Didagelos and Gkiouliava 8 In this study, the incidence of isolated aberrant right subclavian artery was approximately 0.86% in neonates, with none of them having an aberrant left subclavian artery, similar to the results of previous studies. In the available literature, 25–37% of patients with an aberrant right subclavian artery were diagnosed with Down syndrome, had congenital cardiac defects, or other chromosomal abnormalities.Reference Atanasova, Markov and Pavlova 14 – Reference Rembouskos, Passamonti and De Robertis 16 Neonates with aberrant right subclavian artery in this study did not have congenital malformations, although their birth size was significantly lower than that of non-aberrant right subclavian artery neonates. The cause of smaller for gestational age in neonates with aberrant right subclavian artery is unclear. We offer up the possibility that aberrant right subclavian artery may be concomitant with this minor abnormality in patients with congenital anomaly.
The occurrence of aberrant right subclavian artery in our study was more common in female (60%) than in male (40%) patients, but this difference was not statistically significant. Polguj et alReference Polguj, Chrzanowski and Kasprzak 17 reported that the occurrence of aberrant right subclavian artery was more common in female than in male patients (55.3 versus 44.7%), which was similar to the results reported by Molz & Burri.Reference Molz and Burri 18 We reviewed the literature to explore the gender distribution of aberrant right subclavian artery, and discovered 431 reported cases of aberrant right subclavian artery. Of these cases, aberrant right subclavian artery occurred in 254 female patients (58.9%) and 177 male patients (41.1%). Another study observed female predominance in cases of left aortic arch with aberrant right subclavian artery (female 75% and male 25%), whereas right aortic arch with aberrant left subclavian artery affected male patients slightly more than twice as often as female patients (male 67% and female 33%).Reference Natsis, Didagelos and Gkiouliava 8 , Reference Polguj, Chrzanowski and Kasprzak 17 , Reference Molz and Burri 18
Various diagnostic techniques can be used to both identify and confirm the diagnosis of an aberrant right subclavian artery and its associated CHD, including barium oesophagraphy, transthoracic echocardiography, multi-detector CT, three-dimensional surface rendering magnetic resonance angiography, and angiography. The feasibility of aortic arch anomaly assessment in newborns through the use of transthoracic echocardiography depends mainly on the skill of the sonographer. Experience with cardiac scans may be important in obtaining a detailed evaluation of the cardiovascular system. Echocardiographic findings suggestive or diagnostic for a vascular ring could be obtained in approximately 55% of the cases reported by Kir et alReference Kir, Saylam and Karadas 1 They found that transthoracic echocardiography was highly accurate for diagnosing a pulmonary arterial sling and double aortic arch (100%), but its diagnostic yield for aberrant right or left subclavian artery was lower (40%). Our observations showed that the diagnostic yield of transthoracic echocardiography for the diagnosis of aberrant right subclavian artery could be improved. In our previous study of 42 children with isolated aberrant right subclavian artery,Reference Jan, Lin and Fu 19 we noted that transthoracic echocardiography performed by an experienced echocardiographer can be a highly accurate screening tool for the diagnosis of aberrant subclavian artery in comparison with multi-detector CT and three-dimensional surface rendering magnetic resonance angiography imaging studies. We used multi-detector CT or three-dimensional surface rendering magnetic resonance angiography to confirm the diagnosis and clarify the anatomical relationship between the vessels and trachea/oesophagus for the first 21 patients, and found that this anomaly could be 100% definitively diagnosed by transthoracic echocardiography. The diagnostic techniques of transthoracic echocardiography are described in the Materials and methods section. The key to an accurate diagnosis of an aberrant right subclavian artery using transthoracic echocardiography is the careful delineation of the aortic arch and its branching pattern. Visualisation of the non-bifurcating first branch of the aortic arch coursing as the aortic arch appears contralateral to the carotid artery, and finding two separate arteries of the right common carotid artery and aberrant right subclavian artery towards the right side, serves as a diagnostic clue for an aberrant right subclavian artery. Transthoracic echocardiography is also an important tool for both ruling out and evaluating coexisting cardiac anomalies, as nearly one-fourth of a right-sided aortic arch with aberrant left subclavian artery is associated with CHD.Reference Backer, Mavroudis and Rigsby 5
Therefore, an aberrant right subclavian artery can be found incidentally by transthoracic echocardiographic screening. In clinical practice, extrinsic compression of the airway and oesophagus caused by aberrant right subclavian artery is rare in infancy at least, with clinical manifestations depending on the severity of any oesophageal–tracheal narrowing present. Some patients with an isolated aberrant right subclavian artery may be asymptomatic their entire life, whereas some patients may develop respiratory distress, stridor, wheezing, dyspnoea, recurrent respiratory tract infections, or feeding problems caused by compression of the trachea and/or oesophagus. These symptoms are conventional indications for surgical intervention in patients with an aberrant right subclavian artery.Reference Kir, Saylam and Karadas 1 – Reference Backer, Mavroudis and Rigsby 5 The majority of cases reported in the literature regarding left aortic arch and aberrant right subclavian artery were asymptomatic, whereas patients with right aortic arch with aberrant left subclavian artery often have either a diverticulum of Kommerell or midline descending aorta, along with a higher rate of airway and/or oesophagus compression.Reference Morrow and Huhta 3 , Reference Weinberg 4 However, there are few reports concerning the follow-up of newborns with isolated aberrant right subclavian artery, and it therefore remains unclear whether early surgical intervention is warranted during the infant period if the child is initially asymptomatic. Thus, physicians may find it challenging to follow up infants with an aberrant right subclavian artery. In this study, we found that there were no statistically significant differences in clinical manifestations, including developmental history, gastrointestinal, and respiratory symptoms. Patients were usually asymptomatic, and only a few patients had a history of feeding or respiratory problems, which may have been aberrant right subclavian artery-related. However, the identification of feeding problems during infancy is no simple task as there is no universally accepted definition or classification system. In addition, feeding problems are heterogeneous in nature as illustrated by the following list of symptoms regarding feeding problems in infancy, which may not be aberrant right subclavian artery-related: milk or food dislikes or refusal, difficulty sucking or swallowing, and regurgitation or vomiting. In addition, aetiological factors contributing to feeding problems are often multifactorial and may interact, leading to the final clinical picture of simply “an infant experiencing feeding problems”, without a clear aetiology. Although neonates with isolated aberrant right subclavian artery had a smaller birth size than normal neonates, their growth rates of body length and weight for age during infancy were not significantly different between neonates with and without an isolated aberrant right subclavian artery; however, this needs a larger study and longer follow-up to clarify whether or not there is a real difference. Moreover, recurrent upper airway symptoms are common in infancy, and thus the work-up must include a wide range of differential diagnostic possibilities. Aberrant right subclavian artery-related respiratory symptoms can be caused by direct tracheal compression or bronchial aspiration secondary to oesophageal compression by the structures forming the ring;Reference Turner, Gavel and Coutts 20 however, none of the aberrant right subclavian artery patients in this study experienced a lower respiratory tract infection.
Limitations
There were some limitations in this study. First, there were a low number of cases, which may therefore explain the lack of statistically significant differences in gender, and some clinical manifestations. Further investigation with a larger number of cases remains essential. Second, gastrointestinal symptoms such as vomiting and dysphagia in patients with an aberrant right subclavian artery may develop when solid nutrients are introduced beyond 4–6 months of age. Moreover, lumen compromise may manifest itself years later, such as owing to the presence of a diverticulum of Kommerell at the origin of the aberrant right subclavian artery, and therefore a duration of 1 year for follow-up may not be long enough. Third, although our previous study concluded that a transthoracic echocardiography performed by an experienced echocardiographer can be a highly accurate screening tool for the diagnosis of an aberrant right subclavian artery,Reference Jan, Lin and Fu 19 complete studies including an oesophagram, angiography, CT, and/or MRI were not performed to confirm the diagnosis in this study.
Conclusions
Transthoracic echocardiography can be applied as a first-line investigation tool in the diagnostic screening for an aberrant right subclavian artery. Neonates with isolated aberrant right subclavian artery had a smaller birth size, although there were no significantly different rates of growth in body length and weight when compared with normal healthy infants. This needs a larger study and longer follow-up to clarify whether or not there is a real difference. This study does not support an active surgical policy for asymptomatic infants with isolated aberrant right subclavian artery. A larger study and longer follow-up of affected infants is recommended.
Acknowledgements
The authors thank Mr Peter John Wilds and Mr Dean Spencer Dowers for their assistance with the English language editing of the manuscript. The authors are also grateful to all of our colleagues in the Division of Pediatric Cardiology, Children’s Medical Center, Taichung Veterans General Hospital, for their help.
Financial Support
This work was supported by the Taichung Veterans General Hospital, Taiwan, under grant TCVGH-1056506B.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this study comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees.







