Atrial septal defect is one of the most common congenital heart diseases. Surgical correction under cardiopulmonary bypass and transcatheter device closure of atrial septal defect are the main methods for treating atrial septal defects. Reference Yi, Guo and You1,Reference Chen, Cao and Chen2 In recent years, transcatheter device closure of atrial septal defects guided solely by transthoracic echocardiography has been widely applied in China. Reference Zhu, Qiang, Liu, Xie, Zheng and Sun3–Reference Amoozgar, Soltani and Edraki5 In most adult patients, transcatheter device closure of atrial septal defects guided by fluoroscopy and echocardiography can be performed under local anesthesia combined with proper sedation. However, it has not been reported whether transcatheter device closure of atrial septal defects guided solely by transthoracic echocardiography in adult patients can also be completed during conscious sedation combined with local anesthesia in the operating room. Because an aseptic sheet covered the head and face, it was inconvenient for observation and respiratory management, and strict braking was needed during the procedure, so it put forward higher requirements for perioperative controllability and safety sedation and analgesics. The purpose of this study was to investigate the feasibility and safety of conscious sedation with midazolam in transcatheter device closure of atrial septal defects guided solely by transthoracic echocardiography.
Materials and methods
The study was examined and approved by the ethics committee of Fujian Medical University, and all patients signed informed consent forms. There were 55 patients with conscious sedation who underwent transcatheter device closure of atrial septal defects under the sole guidance of transthoracic echocardiography between October, 2019 and May, 2020 in this study. A group of previous patients with unpublished data who underwent the same procedure with general anesthesia was set as the control group (Table 1). All patients underwent routine pre-operative examinations, such as electrocardiogram, chest X-ray, transthoracic echocardiography, etc. The inclusion criteria were a simple and secundum-type atrial septal defect with left-to-right shunt was present; the diameter of the defect was 30 mm, and the distance from the edge of the atrial septal defect to the surrounding structure was 5 mm, determined by transthoracic echocardiography; and the patient was willing to undergo a transcatheter procedure and agreed to the anesthetic regimen. The exclusion criteria were multiple or ostium primum defect; an atrial septal defect combined with endocarditis and hemorrhagic diseases; presence of thrombus at the placement of the occluder and venous thrombosis at the insertion of the catheter; right-to-left shunt caused by severe pulmonary hypertension; patients with other cardiac malformations requiring surgical treatment; and patients with severe arrhythmia or heart failure.
Table 1. Pre-operative and perioperative parameters between the two groups
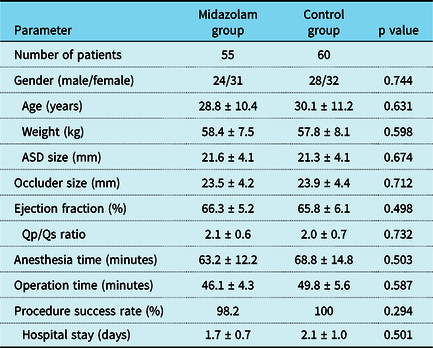
Abbreviations: ASD = atrial septal defects.
All patients were fasted for 6–8 hours and refrained from drinking water for 2˜4 hours before the operation. After entering the operating room, the electrocardiogram and pulse oxygen saturation (SpO2) were monitored. The peripheral venous pathway was established, and continuous infusion of normal saline was given. Radial artery puncture was performed to monitor arterial blood pressure directly, and subclavian venipuncture was performed to monitor central venous pressure. In the midazolam group, all patients received an intravenous infusion of midazolam 0.05 mg/kg and fentanyl citrate 1 mg/kg during anesthesia induction and then received injection-pump midazolam 15 µg/kg/hour until satisfactory sedation was achieved. All patients were given oxygen at a rate of 2 L/minute through a mask. The infusion of sedatives was stopped after the release of the atrial septal defect occluder. In the control group, all patients received routine general anesthesia with endotracheal intubation and mechanical ventilation. During the operation, the sedative dose was adjusted according to the depth of anesthesia, and the bispectral index (BIS) value was maintained at 40–60. The time from the administration of sedative painkillers to the removal of the vascular sheath is the time of anesthesia. The time from the establishment of venous access to the removal of the vascular sheath was the time of operation.
Patients were placed in the supine position, and 1 mg/kg heparin was given intravenously. Local anesthesia with 2% lidocaine was injected into the puncture point. Before the operation, the diameter of atrial septal defect and the size of the occluder were confirmed. Because of the selective enrolment of patients, cardiac catheterization was not routine in this study. Then, the right femoral vein was punctured, and the sheath was implanted. Under the guidance of an apical four-chamber view, the catheter and guidewire were inserted into the inferior caval vein, then sent from the right atrium to the left atrium through the atrial septal defect. The delivery track was established and the occluder was released through a delivery sheath. After the operation, the patient was sent back to the general ward for further monitoring and treatment.
The related clinical data were recorded, including age, gender, body weight, size of atrial septal defect, size of the occluder, anesthesia time, time of operation, and length of hospital stay. Central venous pressure, mean arterial pressure, heart rate, blood oxygen saturation, and BIS score were also monitored. Respiratory inhibition, intraoperative awareness, nausea, and vomiting were observed during and after treatment, and other post-operative complications were recorded. After the patients’ consciousness was restored, the nurses calculated the patient’s sedation and analgesia scores three times in a row every 30 minutes. One week after discharge, the patients were followed up and asked to complete a satisfaction questionnaire survey.
The Ramsay sedation score was used to measure the degree of sedation in patients, and the scoring standardization was as follows: anxious or restless, or both were scored as 1; cooperative, oriented and tranquil were scored as 2; response to commands was scored as 3; brisk response to the stimulus was scored as 4; sluggish response to the stimulus was scored as 5; and no response to the stimulus was scored as 6. A score of 1 point indicated a lack of sedation, 2–4 points indicated appropriate sedation, and 5–6 points indicated excessive sedation. Reference Morelli, Ierardi and Biondetti6,Reference Zhou, Liu, Tan, Ji, Yi and Song7
The numerical rating scale was used to measure analgesia in patients. The NRS allowed a person to describe the intensity of his/her pain as a number usually ranging from 0 to 10, where “0” meant “no pain” and “10” meant pain as “bad as it could be,” and the patient circled a number to represent the degree of pain. We regarded 0 as no pain, 1–3 as mild pain, 4–6 as moderate pain, and 7–10 as severe pain. Reference Park, Hwang and Cho8,Reference Han, Lee, Ha and Kim9
The Patient Satisfaction Questionnaire-18 scale was used to evaluate patient satisfaction. The Patient Satisfaction Questionnaire-18 contained 18 items, covering seven dimensions of medical service satisfaction: general satisfaction, technical quality, interpersonal manner, communication, financial aspects, time spent with doctor accessibility and convenience, accessibility, and convenience. Each item used a five-point Likert scale, with a score of 1–5. The higher the score, the higher the satisfaction was. The scores of these subscales were added together to form an overall satisfaction score. Reference Davtyan, Aghabekyan, Davtyan, Hayrapetyan and Aslanyan10–Reference Ameh, Gómez-Olivé, Kahn, Tollman and Klipstein-Grobusch12
The data of this study were analyzed with SPSS 22.0. Continuous data are summarised as the mean ± standard deviation, whereas categorical data are summarised as percentages. The paired t-test was used for continuous variables conforming to the normal distribution test. The Mann–Whitney U test was used to analyze the differences in the Ramsay sedation score, numerical rating scale score, and post-operative satisfaction score, which had non-normal distributions.
Results
There were no significant differences in age, gender, body weight, size of atrial septal defect, size of the occluder, time of anesthesia, time of operation, and length of hospital stay, which indicated that the two groups of patients were homogeneous and comparable (Table 1). In the midazolam group, a total of 55 patients aged between 16 and 58 years underwent transcatheter device closure of atrial septal defects with a 98.2% success rate. Occluder dislodgement occurred in one patient transferred for surgical repair due to the insufficient rim of the inferior caval vein. There was hemodynamic stability during the procedure in the midazolam group, and none of the patients needed endotracheal intubation for general anesthesia. No anesthesia-related and other device-related complications were observed. Compared with the control group, the midazolam group had no statistically significant differences in the Ramsay sedation score and numerical rating scale scores (Table 2). Patients in the midazolam group experienced more post-operative satisfaction than patients in the control group (Table 3).
Table 2. Sedation and analgesia scores between the two groups

Abbreviations: NRS = the numerical rating scale; RSS = the Ramsay sedation score.
Table 3. PSQ-18 scale scores between the two groups

PSQ-18 = Patient Satisfaction Questionnaire 18. Scores represent the score on the five-point Likert scale (1 = totally disagree, 2 = disagree, 3 = not sure, 4 = agree and 5 = totally agree).
Discussion
Atrial septal defects are the most common congenital heart diseases, among which secundum atrial septal defects are the most common type. Reference Rouatbi, Farhat, Heying, Gérard, Vazquez-Jimenez and Seghaye13,Reference Berthoud, Ennezat and Guerbaai14 Surgical repair and transcatheter device closure guided by fluoroscopy and echocardiography have been the main treatments for atrial septal defects, and the two methods have their advantages and disadvantages. Reference Bonatti, Vetrovec, Riga, Wazni and Stadler15–Reference Roguin, Goldstein, Bar and Goldstein18 In recent years, transthoracic device closure with a mini-incision and transcatheter device closure guided solely by transthoracic echocardiography has been widely used in China. In this study, transcatheter device closure of atrial septal defects guided solely by transthoracic echocardiography could avoid radiation exposure to both doctors and patients, avoid chest incisions, do not require cardiopulmonary bypass, and simplify the operation process. Reference Chen, Cao, Zhang, Chen, Lu and Yu19 Besides, because it can be carried out in the operating room, if the operation failed, it could be immediately converted to surgical correction to ensure patients’ safety.
The critical factor of our method was the accurate evaluation of the location, size, and rims of the atrial septal defect under the guidance of transthoracic echocardiography and the establishment of the delivery track. Among the 55 patients included in the midazolam group, only one patient was transferred for surgical repair because of an insufficient rim of the inferior caval vein. While pre-operative transthoracic echocardiography diagnosed an atrial septal defect with sufficient inferior vena cava (IVC) rim in this case, the final diagnosis was confirmed as an atrial septal defect with deficient IVC rim by surgical operation and transesophageal echocardiography. These results demonstrated that transesophageal echocardiography was more advantageous than transthoracic echocardiography in the diagnosis of atrial septal defect. For patients with unclear diagnosis, pre-operative computed tomography angiography examination would be performed. Besides, intraoperative transesophageal echocardiography would be performed to determine whether there was any indication for device closure. In our experience, with the help of experienced cardiac sonographers and a satisfactory echo window, transthoracic echocardiography could also be used as a guiding tool for device closure of atrial septal defect. Reference Chen, Cao, Zhang, Chen, Lu and Yu19 For patients with unclear echo window and suitable cooperation, transesophageal echocardiography was adopted for the diagnosis, and transthoracic echocardiography was used for guidance. In the rest of the patients, the echo window was right for completing the procedure.
Ding and this team showed that transcatheter device closure of atrial septal defect by transthoracic echocardiography guidance alone in selected adults might be considered efficacious and safe as transesophageal echocardiography guidance. Reference Ding, Chang, Lin, Wu and Hsieh20 Oto and his colleagues concluded the safety and efficacy of transcatheter device closure of patent foramen ovale under transthoracic echocardiography guidance, resulting in shortened procedural time without the need for endotracheal intubation or general anesthesia. Reference Oto, Aytemir and Ozkutlu21 The use of transthoracic echocardiography guidance during the operation was more non-invasive than the use of transesophageal echocardiography, which could avoid such injury factors associated with general anesthesia, endotracheal intubation, and esophageal ultrasound probes and reduce the operation time. Reference Dou, Kan and Guo22 Because of the short operation time and lack of need for general anesthesia, the use of short-acting anesthetics for conscious sedation might be more suitable for this procedure.
Studies have shown that the level of sedation required for transcatheter device closure of atrial septal defects is conscious sedation or moderate sedation. Reference Pan, Xie, Zhang, Long, Xu and Zhang23 During this sedation process, the patients’ respiratory and cardiovascular functions were stable, and they had apparent responses to auditory and tactile stimuli. Conscious sedation was also conducive to the early detection of neurological signs and other complications during the procedure. Reference Kapoor, Sharma, Sharma, Dugal and Singh24 Other potential advantages of conscious sedation were that patients could be woken up faster, post-operative anesthesia-related complications were fewer, and the early post-operative rehabilitation effect was better.
Midazolam is a water-soluble benzodiazepine that provides short-term sedation by inhibiting the central nervous system. If opioids are used together with midazolam, the amnesia symptoms of patients can be relieved, the effects of analgesia and sedation can be improved, and patients’ comfort and satisfaction can be higher. Reference Soong, Afifi and McGee25 Fentanyl was combined with midazolam during anesthesia induction in this study. Studies had shown that when midazolam was used in combination with opioid painkillers, the dosage of midazolam and opioids might be reduced because of the synergistic effect. Reference Ji, Liu and Xue26 To avoid respiratory depression, patients with cardiopulmonary diseases should be given low doses of fentanyl and midazolam. Reference Yao, Su and Chen27–Reference Wang, Zhang, Huang and Peng29 There was no respiratory depression in this study, and no patient needed endotracheal intubation. The SpO2 of all patients was maintained at more than 95% during the operation.
During the operation, blood pressure, heart rate, central venous pressure, BIS value, and oxygen saturation were closely monitored; all of them fluctuated slightly within the normal range. After the operation, the sedation and analgesic effects of the patients were not affected by different anesthesiologists and surgeons, and the scores of all patients were in the range of mild pain. Compared with the control group under general anesthesia, there was no significant difference in analgesia and sedation among the midazolam group patients, indicating that good results could be achieved with the combination of midazolam and local anesthesia. The post-operative follow-up results showed that the patients in the midazolam group were more satisfied with the operation process than patients in the control group. There was an absence of endotracheal intubation, mechanically assisted ventilation, ICU stay, and potentially lower hospitalization costs, which indicated that this anesthesia protocol had high safety, a good sedative effect, and high patient comfort in patients.
There were still some limitations to this study. First, this study was a retrospective study involving a small sample size. Second, the patients participating in this study were relatively healthy. Therefore, the conclusions may not be extended to open-heart surgery under cardiopulmonary bypass and some patients with poor general conditions. Third, there was no literature to support the use of conscious sedation in transcatheter device closure in children and mentally disabled patients. Those patients have low active cooperation ability. During the conscious sedation process, they could not cooperate reasonably with the doctor to complete the operation, enhancing the patient’s feelings of resistance and fear. Although a total of five patients with the age of 16–18 were included in the midazolam group, they were all able to complete the coordination. We still recommend that pediatric patients and mentally disabled patients might not be eligible for this technique. Fourth, we did not compare fentanyl with some other opioids, nor did we determine the optimal dose. Finally, a prospective, randomised, double-blind clinical trial needs to be conducted.
Conclusion
Conscious sedation using a midazolam-based anesthesia regimen and lidocaine-based local anesthesia is a safe, effective, and satisfactory anesthetic technique for transcatheter device closure of atrial septal defects guided solely by transthoracic echocardiography. This scheme is worth popularizing.
Acknowledgements
We highly acknowledge the contribution by the participating doctors: Dao-Zhong Chen, Feng Lin, Qi-Min Wang, Han-Fan Qiu, Xue-Shan Huang, Dong-Shan Liao, Xiao-Fu Dai, and Gui-Can Zhang from Union Hospital, Fujian Medical University. We hope humans eventually defeat COVID-19.
Conflict of interests
None.
Financial support
This study was supported by the National Key Research and Development Program of China (grant no. 2016YFC1301900).






