Case report
A 24-year-old multigravida was referred for fetal echocardiogram at 33 weeks 5 days gestation after a growth ultrasound revealed a narrowed ductus arteriosus, dilated and poorly functioning right ventricle, and tricuspid regurgitation. Pregnancy course to date was uncomplicated with a normal 20-week anatomy scan and non-contributory obstetric history. She admitted to taking BC powder® (acetylsalicylic acid 1000 mg, caffeine 65 mg) the prior month but denied use of other medications.
Fetal echocardiogram revealed severe ductal constriction with turbulent flow by colour Doppler (Figure 1), elevated peak systolic (3.34 m/s; normal 0.50–1.40 m/s) and end-diastolic (1.71 m/s; normal 0.06–0.30 m/s) (Figure 2) Doppler velocities with decreased pulsatility index (0.76; normal 1.9–3), moderate right atrial and right ventricular dilation, moderate right ventricular hypertrophy, monophasic right ventricular inflow, severe holosystolic tricuspid regurgitation with the jet reaching the back wall of the right atrium and peak velocity of 4.6 m/s, severely depressed right ventricular global function with elevated Tei index (1; normal 0.35 ± 0.07), and decreased shortening fraction (13%; normal 34 ± 3%).Reference Huhta, Moise and Fisher1–Reference Bader, Hornberger and Huhta4 Two-dimensional sagittal imaging of the ductus arteriosus diameter measured 0.26 cm (z-score −3.4). The fetus was in normal sinus rhythm and hydrops was absent. Patient was admitted for further management.
Inpatient continuous cardiotocography was Category 1 and corticosteroids were administered in anticipation of possible premature delivery. Daily obstetric ultrasounds documented normal amniotic fluid index and no hydrops. Oral Digoxin 500 mcg was given twice a day for 2 days, followed by 250 mcg twice a day for the remainder of pregnancy. Following the fourth dose, serum level was 1.8 ng/ml (goal of 1–2 ng/ml). Oxygen was delivered through non-rebreather mask at 12 L/minute, with 1-hour “breaks” every 8 hours using 4 L/minute nasal cannula. Maternal baseline and post-load electrocardiograms were normal. Fetal echocardiogram on day 1 was unchanged. By day 3, there were decreased ductal velocities with peak systolic velocity 2.43 m/second and end-diastolic velocity 0.48 m/second, increased pulsatility index of 1.5, mild right atrial and right ventricular dilation, moderate right ventricular hypertrophy, biphasic right ventricular inflow, decreased tricuspid regurgitation jet with peak systolic velocity of 2.1 m/second, and improving right ventricular function (Tei index 0.39, shortening fraction 25%). Ductal diameter remained constricted. She was discharged on day 4 on 4 L/minute oxygen via nasal cannula, oral Digoxin 250 mcg twice a day, and with close follow-up.
The patient complied with prescribed therapeutics and reported no adverse effects. Semiweekly outpatient fetal echocardiograms demonstrated progressively reduced ductal peak systolic and end-diastolic gradients, increasing pulsatility index, normalisation of right ventricular size and contractility (Tei index 0.33, shortening fraction 32%), and improved tricuspid valve inflow with trivial regurgitation jet. Ductal diameter remained unchanged. Weekly biophysical profile scores were 8/8, without hydrops.
Preterm premature rupture of membranes necessitated labour induction at 35 weeks 4 days gestation. She vaginally delivered a 2710 g male neonate, Apgars 9 and 9 at 1 and 5 minutes. Given prematurity, he was admitted to the Neonatal ICU. Immediate postnatal echocardiogram showed qualitatively normal right ventricular size and function, trivial tricuspid regurgitation, and unrestricted patent ductus arteriosus with bidirectional flow. Echocardiogram 2 days later showed ductal closure with no secondary evidence of pulmonary hypertension and normal biventricular function. He was discharged home on day of life 4 with no sequelae.
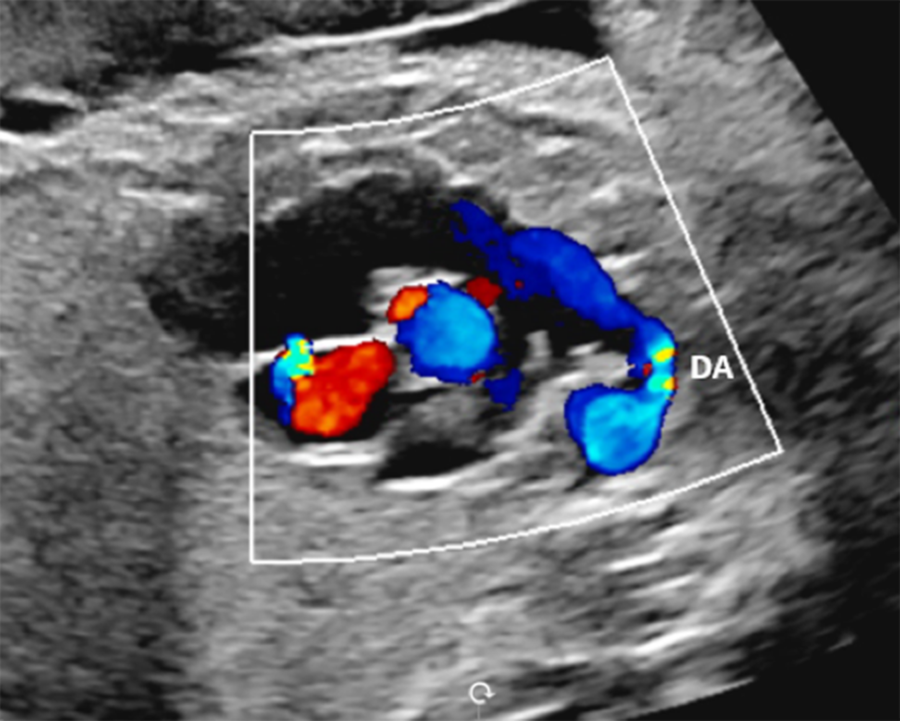
Figure 1. Sagittal image demonstrating the constricted ductus arteriosus.
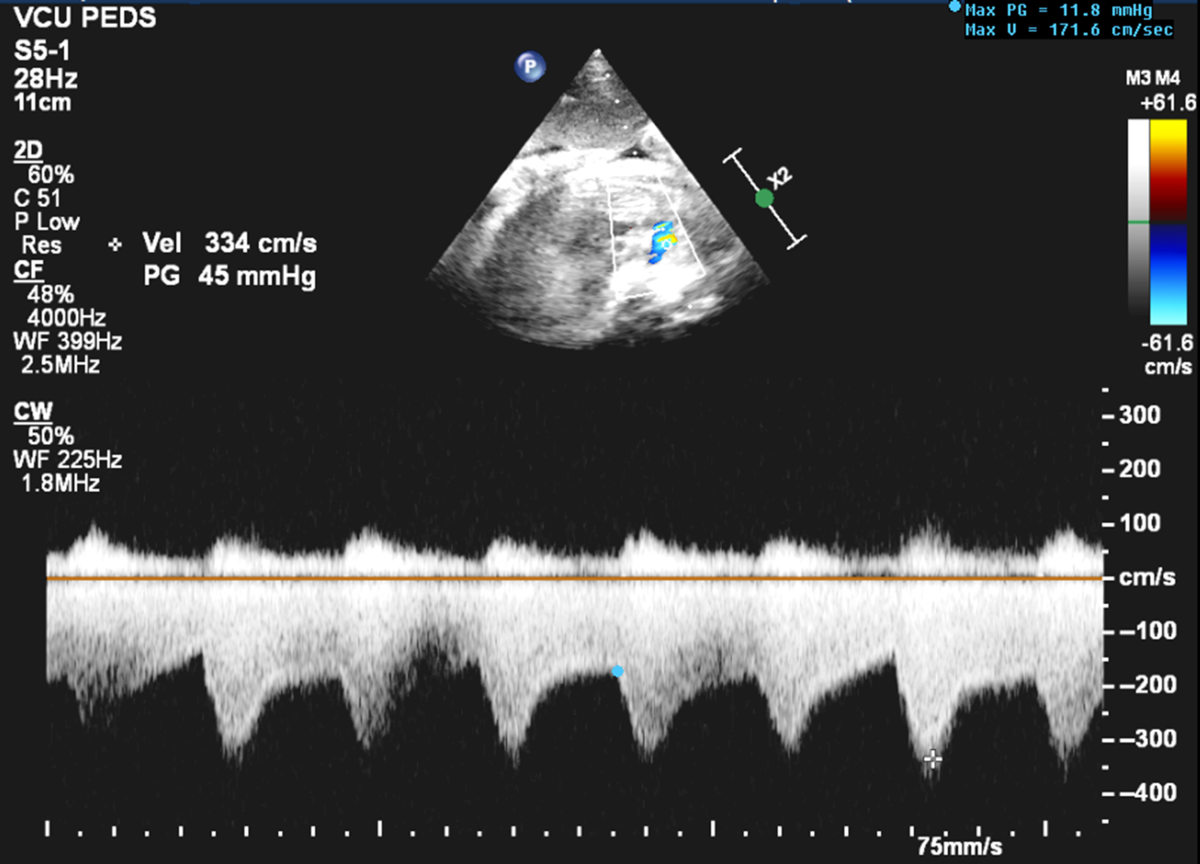
Figure 2. Continuous wave Doppler of the ductus arteriosus showing increased peak systolic and end-diastolic velocities at 33 weeks 5 days gestation.
Discussion
Antenatal constriction of the ductus arteriosus is rare, occurring predominantly secondary to prostaglandin synthase inhibitors, CHD, and idiopathic causes. In this patient, the constriction may have been secondary to the non-steroidal anti-inflammatory drug component of BC powder®.Reference Mielke and Benda2,Reference Ishida, Inamura, Kawazu and Kayatani5 Maternal Digoxin and oxygen therapy decreased pulmonary vascular resistance, attenuated ductal flow, and improved right ventricular function as seen by the reduced ductal Doppler velocities and normalisation of the right ventricular Tei index and shortening fraction. This resulted in a healthy newborn with normal right ventricular function and without persistent pulmonary hypertension on echocardiogram.
The ductus arteriosus connects the pulmonary artery to the descending aorta.Reference Heymann and Rudolph6 Ductal flow in-utero is from right to left and biphasic, with a normal peak systolic velocity of 0.50–1.40 m/second, end-diastolic velocity of 0.06–0.30 m/second, and pulsatility index of 1.9–3.Reference Huhta, Moise and Fisher1,Reference Mielke and Benda2 Approximately 90% of right ventricular output passes through the low-resistance ductus arteriosus; the remainder enters the high-resistance pulmonary vasculature.Reference Heymann and Rudolph6,Reference Smith7 Low arterial partial pressure of oxygen and circulating prostaglandins maintain ductal patency in-utero.Reference Heymann and Rudolph6,Reference Smith7
With ductus arteriosus constriction, blood intended to cross the ductus is redirected into the high resistance pulmonary circulation, thus exposing the right ventricle to increased afterload.Reference Heymann and Rudolph6 The right ventricle and vascular media of the pulmonary arteries can both hypertrophy, with resulting right heart failure, fetal hydrops and/or intrauterine death, or postnatal pulmonary hypertension.Reference Huhta, Moise and Fisher1,Reference Ishida, Inamura, Kawazu and Kayatani5,Reference Arunamata, Axelrod, Bianco, Balasubramanian, Quirin and Tacy8
Digoxin for heart failure treatment improves cardiac inotropy by inhibiting ATPase, thereby increasing calcium in heart muscle. Oxygen, a known pulmonary vasodilator, decreases pulmonary vascular resistance, thereby decreasing right ventricular afterload. Although maternal hyperoxygenation in late gestation may cause ductal constriction, given the severity of constriction, we aimed to maximise the known drop in pulmonary vascular resistance with hyperoxygenation to promote antegrade pulmonary blood flow and preserve right ventricular function.Reference Huhta, Moise and Fisher1,Reference Arunamata, Axelrod, Bianco, Balasubramanian, Quirin and Tacy8 Oxygen's vasodilatory effect, in combination with Digoxin’s inotropic effect, are the proposed mechanisms for improved right ventricular function in this case.
To our knowledge, the combination of Digoxin and oxygen has not been used for antenatal management of ductus arteriosus constriction. A PubMed literature search from 1980 to present using keywords “constricted ductus arteriosus”, “Digoxin”, and “oxygen” showed two case reports of Digoxin use and no reports of hyperoxygenation with a constricted ductus arteriosus.Reference Harlass, Duff, Brady and Read9,Reference Mielke, Steil and Gonser10 Harlass et al described a case of ductus arteriosus closure resulting in fetal heart failure with severe oligohydramnios, ascites, and anasarca.Reference Harlass, Duff, Brady and Read9 Digoxin was used to treat heart failure; however, fetal death occurred. Mielke et al described using Digoxin for fetal atrial flutter with idiopathic ductus arteriosus stenosis.Reference Mielke, Steil and Gonser10 Maternal hyperoxygenation is theorised to be beneficial in other CHD, such as Ebstein’s anomaly, by reducing pulmonary vascular resistance and promoting antegrade pulmonary blood flow.Reference Arunamata, Axelrod, Bianco, Balasubramanian, Quirin and Tacy8
Based on our report and literature review on management of severe ductus arteriosus constriction, we propose that antenatal management with maternal Digoxin and continuous oxygen therapy may improve fetal right ventricular function, decrease postnatal pulmonary hypertension, and improve neonatal outcomes.
Acknowledgments
We would like to thank Dr. Lisa Hornberger and Dr. Angela McBrien at Stollery Children's Hospital in Edmonton, Canada for their helpful contributions to our case report.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.




