Published online by Cambridge University Press: 21 January 2005
Acquired lengthening of the QT interval due to hypocalcaemia is a rare cause of arrhythmia in childhood. Early recognition, rapid institution of appropriate cardiac monitoring, and replacement therapy are essential. An endocrinal work-up may be necessary to exclude primary disorders of calcium metabolism. We report four cases documenting the varied clinical spectrum in which hypocalcaemic-induced prolongation of the QT interval and arrhythmia can occur in childhood.
Hypocalcaemia prolongs the duration of the second, or plateau, phase of the cardiac action potential, manifesting as an increased duration of the ST segment, and consequently the QT interval.1 Prolongation of repolarisation, the consequent delay in inactivation of calcium ion channels, and the resulting late inflow of calcium, may all contribute to the formation of early after-depolarisations. These may reach the threshold for depolarising adjacent cells, triggering ventricular arrhythmia, and in particular torsade de pointes, so named for its characteristic appearance of QRS complexes of changing amplitude that appear to “twist” around the isoelectric line. The electrocardiographic features of torsade de pointes include labile QT intervals, prominent U waves, and a “pause-dependent” onset of the arrhythmia.2–4 Here we report four cases documenting the varied clinical spectrum in which hypocalcaemic-induced prolongation of the QT interval and arrhythmia can occur in childhood.
A 24-week premature male presented with persistent bradycardia on the first day of life due to “pseudo” 2:1 atrioventricular block (Fig. 1a). A 12-lead electrocardiogram demonstrated a corrected QT interval of 545 milliseconds. Serum electrolytes revealed low total calcium, at 1.6 millimoles per litre, with the normal values for age in our laboratory being 1.87 to 2.49 millimoles per litre. Levels of magnesium were normal, but potassium was high at 6.71 millimoles per litre, with our normal values being 3.5 to 5.5 millimoles per litre. During an intravenous infusion of calcium, he developed 3:2 Wenckebach atrioventricular block, and then converted to a “wide complex” tachycardia with marked acute shortening of the QT interval (Fig. 1b). The QRS complex also gradually narrowed, revealing 1:1 atrioventricular conduction consistent with sinus tachycardia at the same rate as the rhythm of the wide complex (Fig. 1c). An electrocardiogram repeated 24 hours later demonstrated a normal corrected QT interval of 440 milliseconds, with serum electrolytes in the normal range.

Figure 1. (a) “Pseudo” 2:1 atrioventricular block due to acquired long QT (1st sinus beat conducted, 2nd sinus beat blocked). (b) During intravenous calcium infusion, “pseudo” 3:2 Wenckebach atrioventricular block due to prolonged QT (1st sinus beat conducted, 2nd sinus beat conducted with longer PR and QRS aberrancy, 3rd sinus beat blocked) and then conversion to a “wide complex” tachycardia with marked acute shortening of the QT. (c) With continuous monitoring, wide complex tachycardia gradually narrowed to reveal sinus tachycardia with 1:1 conduction. (d) Sinus bradycardia with junctional escape and ventricular bigeminy [isochronic dissociation] and prolonged corrected QT interval of 645 milliseconds. (e) Brief 7 beat run of torsade de pointes.
A 13-year-old girl complained of palpitations, chest pain, and dizziness 12 hours after receiving her first dose of Daunorubicin during induction therapy for acute promyelocytic leukaemia. A normal corrected QT interval had been previously documented. Preventative treatment for tumour lysis syndrome, including oral allopurinol, intravenous hydration and alkalinisation with intravenous sodium bicarbonate, had been commenced 2 days earlier. Cardiac monitoring revealed a periodic wide complex tachycardia, with a QT interval that appeared prolonged. A 12-lead electrocardiogram demonstrated sinus bradycardia with junctional escape, ventricular bigeminy, and confirmed a prolonged corrected QT interval of 645 milliseconds (Fig. 1d). She then had a brief run of 7 beats showing torsade de pointes (Fig. 1e). Serum electrolytes revealed low total calcium, at 2.07 millimoles per litre, with our normal values for this age group being 2.20 to 2.64 millimoles per litre. Levels of magnesium were low at 0.70 millimoles per litre, with our normal values ranging from 0.74 to 0.99 millimoles per litre, and potassium was low at 2.5 millimoles per litre. Her levels of calcium, magnesium and potassium were all normalised with intravenous supplements, and her rhythm stabilised. Her corrected QT interval, which initially remained intermittently prolonged with diffuse abnormalities of the ST segments and T waves, and occasional premature ventricular complexes, subsequently normalised.
A 13-year-old boy was admitted to hospital with convulsive syncope, and a recent history of increasing exercise-induced carpopedal spasm, lethargy, headaches, emotional lability, facial muscle twitching and hyperacusis. He had a past history of photophobia, dental crowding and malalignment, and recurrent fungal infections. There was a family history of seizure disorders and hypothyroidism, but no exertional syncope or sudden death, or any other endocrinal disorders. His Chvostek's and Trousseau's signs were both negative, and he had no ankle clonus. A 12-lead electrocardiogram demonstrated a prolonged corrected QT interval of 520 milliseconds (Fig. 2a). Serum electrolytes revealed low total calcium at 1.13 millimoles per litre, high levels of phosphate at 3.08 millimoles per litre, with our normal values ranging from 1.07 to 1.74 millimoles per litre, and low values for magnesium, at 0.50 millimoles per litre. On endocrinal work-up, levels of parathyroid hormone were less than 0.7 picomoles per litre, normal values being from 0.7 to 7.0 picomoles per litre. Calcifications were noted in the basal ganglia on computerised tomographic scanning, and keratopathy was diagnosed on an ophthalmologic review. We made the diagnosis of hypoparathyroidism due to polyglandular autoimmune syndrome, type 1, and this was subsequently confirmed on molecular genetic testing. His symptoms resolved with supplemental calcium and calcitriol, and his corrected QT interval normalised (Fig. 2b).
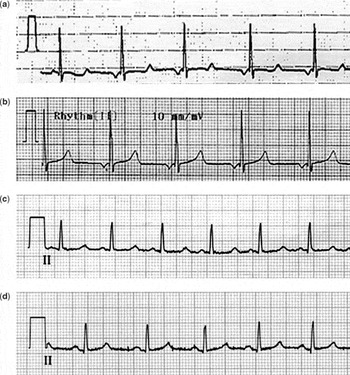
Figure 2. (a) Low atrial rhythm with prolonged QTc of 520 milliseconds. (b) Normalised QTc at 440 milliseconds. (c) Normal sinus rhythm with prolonged QTc of 490 milliseconds. (d) Normalised QTc of 410 milliseconds.
A 15-year-old girl presented with headache, presyncope and visual symptoms, and a recent history of a single brief episode of sudden loss of consciousness, “tingling toes”, and intermittent stress urinary incontinence. She had a past history of delayed gross motor milestones, learning difficulties, missing teeth, delayed formation of enamel, asthma, and eczema. There was a family history of vasovagal syncope and hypothyroidism, but no exertional syncope or sudden death, or other endocrinal disorders. Her Chvostek's and Trousseau's signs were positive, her metacarpals appeared normal, and she had no ankle clonus. A 12-lead electrocardiogram demonstrated a prolonged corrected QT interval of 490 milliseconds (Fig. 2c). Serum electrolytes revealed low total calcium at 1.29 millimoles per litre, high phosphate at 2.38 millimoles per litre, and low magnesium at 0.63 millimoles per litre. On endocrinal work-up, she was found to have an elevated level of parathyroid hormone at 51 picomoles per litre, and normal levels of 25-hydroxy vitamin D. Calcifications in the basal ganglia and along the junctions of the grey and white matter were also found on a computerised tomographic scan. A diagnosis of pseudohypoparathyroidism, Type 1b, was made, and her symptoms have also resolved with supplementation of calcium and calcitriol. Her corrected QT interval has now normalised (Fig. 2d).
The reason for the hypocalcaemia in our first case, a sick premature neonate, was never elucidated. Infants with congenital long QT syndrome and hypocalcaemic-induced acquired long QT syndrome have been reported as presenting with 2:1 and Wenckebach-type atrioventricular block.5, 6 With sufficient prolongation of the QT interval, the P-wave may fall within the previous T-wave, and not be conducted during the effective ventricular refractory period. Given that hypocalcaemia is relatively common in premature neonates, perhaps consideration should be given to recording more frequently 12-lead electrocardiograms in this clinical setting, looking for prolongation of the QT interval.
Acute anthracycline toxicity was considered as a contributing factor in the second case. The occurrence of hypocalcaemia in the setting of tumour lysis syndrome highlights several important points. First, the reasons for ventricular arrhythmia in the sick child may be multifactorial. Second, electrolytic disturbances rarely exist in isolation. Third, significant electrolytic disturbances may occur in tumour lysis syndrome even with the early instigation of preventative therapy, and warrant cardiac monitoring and frequent monitoring of serum electrolytes.
Although not documented, the syncopal symptoms in the last two cases were potentially precipitated by low cardiac output due to ventricular arrhythmia in the setting of significant prolongation of the QT interval secondary to severe hypocalcaemia. Hypocalcaemic-induced prolongation of the QT interval due to hypoparathyroidism has been reported previously in a 7-year-old boy with autoimmune polyglandular syndrome type I, but with no syncopal symptoms, and in an elderly woman subsequent to the surgical removal of a parathyroid adenoma who developed rate-dependent prolongation of the QT interval and torsade de pointes.7, 8 There is also a previous report of a 12-year-old girl with recurrent exercise induced syncope who had prolongation of the QT interval associated with pseudohypoparathyroidism and hypocalcaemia.9
Any child presenting with syncope, unexplained “seizures”, or near-miss sudden death should have a detailed history taken and a 12-lead electrocardiogram performed. A family history of exertional syncope and sudden death and exposure to agents that prolong the QT interval should always be excluded. Our four case reports highlight the additional need for early recognition of any disease-related electrolytic disturbance. They also demonstrate the importance of rapid institution of appropriate cardiac monitoring and replacement of electrolytes to prevent potentially lethal arrhythmias. When the aetiology of hypocalcaemia is not immediately apparent, early consideration should be given to an endocrinal work-up to exclude primary disorders of calcium metabolism.
We thank Dr Rob Gow, Dr Martin Hosking, Dr Jim Potts and Dr Paul Thiessen for assisting in the preparation of this manuscript.

(a) “Pseudo” 2:1 atrioventricular block due to acquired long QT (1st sinus beat conducted, 2nd sinus beat blocked). (b) During intravenous calcium infusion, “pseudo” 3:2 Wenckebach atrioventricular block due to prolonged QT (1st sinus beat conducted, 2nd sinus beat conducted with longer PR and QRS aberrancy, 3rd sinus beat blocked) and then conversion to a “wide complex” tachycardia with marked acute shortening of the QT. (c) With continuous monitoring, wide complex tachycardia gradually narrowed to reveal sinus tachycardia with 1:1 conduction. (d) Sinus bradycardia with junctional escape and ventricular bigeminy [isochronic dissociation] and prolonged corrected QT interval of 645 milliseconds. (e) Brief 7 beat run of torsade de pointes.

(a) Low atrial rhythm with prolonged QTc of 520 milliseconds. (b) Normalised QTc at 440 milliseconds. (c) Normal sinus rhythm with prolonged QTc of 490 milliseconds. (d) Normalised QTc of 410 milliseconds.