Patent ductus arteriosus is the most frequently seen congenital cardiac lesion in preterm neonates. It occurs in about 30% of neonates younger than 30 weeks of gestational age and up to 60% of neonates younger than 28 weeks of gestational age. Reference Sehgal and McNamara1 Patent ductus arteriosus can have serious clinical consequences in a newborn baby, including pulmonary edema, respiratory insufficiency, heart failure, intracranial haemorrhage, bronchopulmonary dysplasia, necrotising enterocolitis, and death. Reference Hamrick and Hansmann2 More than two thirds of neonates delivered before 28 weeks of gestational age require either pharmacological or surgical closure of the patent ductus arteriosus. Reference Clyman3
The most common surgical approach for the closure of a patent ductus arteriosus is either through a lateral, posterolateral or axillary thoracotomy. However, lung retraction that is necessary in these surgeries can be problematic in a premature patient with over-flowed lungs. Over the past 10 years, we have closed numerous cases of patent ductus arteriosus through an anterior mini-thoracotomy through the second intercostal space in premature infants. In this study, we report our results with this surgical technique by dividing our patients into two groups according to their weight at the time of surgery and comparing the complications and rates of mortality and morbidity of these two groups.
Materials and method
Patient selection criteria
This is a retrospective, observational study which included 103 premature infants (gestational age < 37 weeks) who underwent surgical patent ductus arteriosus ligation between March 2009 and November 2019 at a single institution. Infants who required concomitant procedures or who were not premature at birth were excluded from this study. The surgical patent ductus arteriosus closure was indicated in a premature infant who either remained in congestive heart failure despite medical management or showed a large left-to-right shunt on echocardiograms. All patients had either failed attempts at medical closure with ibuprofen (n = 86) or had contraindications to its use (n = 17). All infants were on mechanical ventilation before the operation. Most operations (n: 84) (82%) were performed in the operating room of our hospital. After the surgery most patients were transferred to the hospital they were referred from. Only three patients (3%) who needed median stermotomy due to complications were cared for in our intensive care unit until they were stable enough for transferal to another institution. The remaining 19 (18%) operations were performed in the intensive care unit of the referring hospital. This study was approved by the Hospital Institutional Ethical Committee. Clinical registration number is 28001928-604.01.01-E.83. The patients were divided into two groups according to their weights at the time of surgery. Data on early postoperative outcomes, 30-day postoperative, and 1-year survival rates were collected and compared between the two groups.
Surgical technique
One transverse incision which length is 2–3 cm was made through the second intercostal space, lateral to the internal thoracic artery. The apex of the lung was gently retracted inferiorly. The pericardium was opened longitudinally, medial and parallel to the phrenic nerve. Stay sutures were inserted and the main pulmonary artery was caudally retracted to visualise the ductus arteriosus and the left and right pulmonary arteries. The lateral surfaces of the ductus were dissected to create a space for a clip. One or two clips were placed on the patent ductus arteriosus (Fig 1). Our surgical technique has been previously published in detail. Reference Karaci, Sasmazel and Turkay4
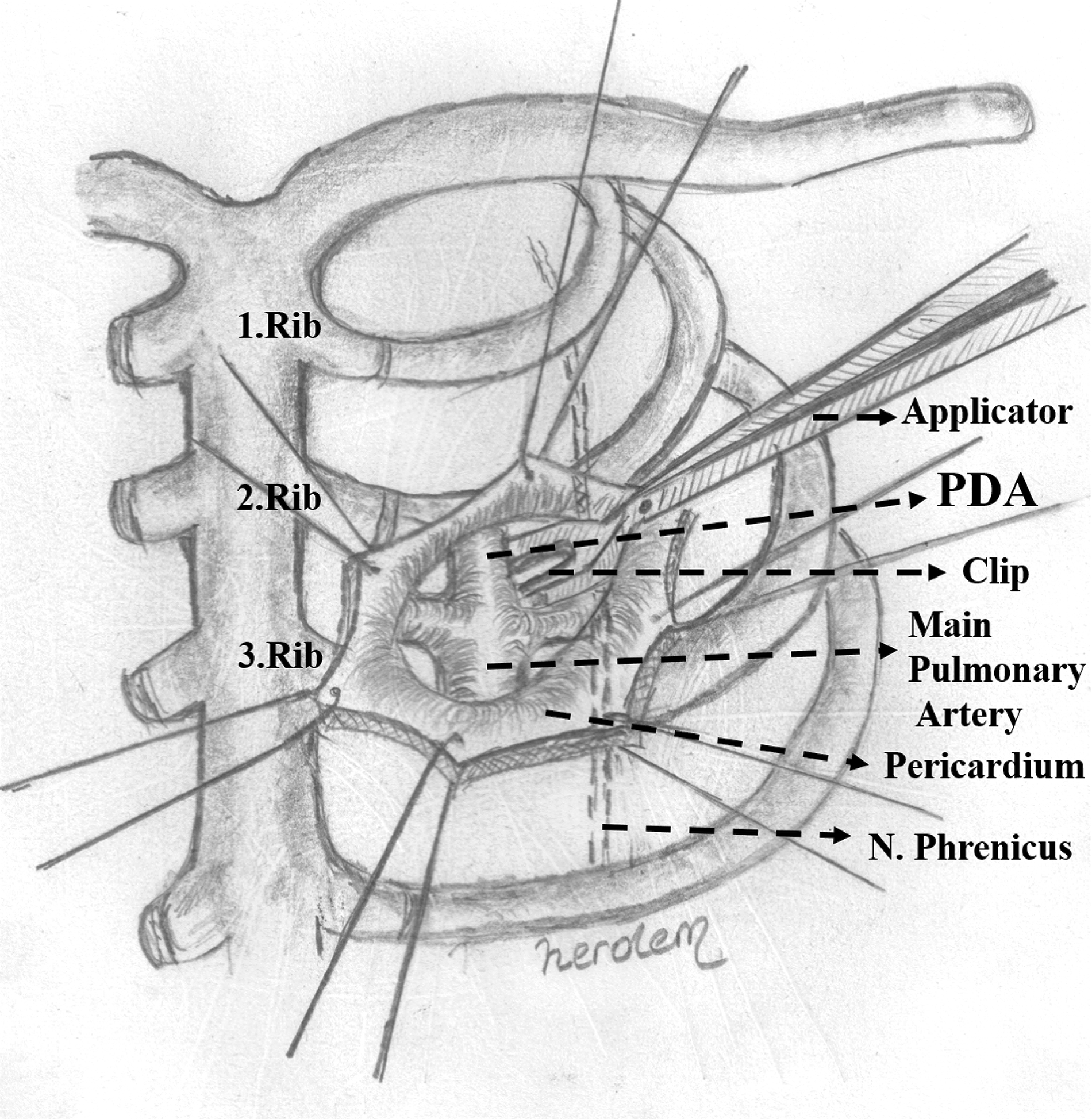
Figure 1. Schematic view of the clip application to patent ductus arteriosus.
(After an anterior, transpleural, mini thoracotomy below the clavicle through the 2nd intercostal space, the apex of the lung is gently retracted inferiorly and laterally. An incision parallel to the phrenic nerve is made on the pericardial surface below the thymus and stay sutures are placed. The main pulmonary artery is caudally retracted to visualise the ductus and the left and right pulmonary arteries. After the lateral surfaces of the ductus are dissected to create a space for a clip. The clip is placed on the patent ductus arteriosus.)
Statistical analyses
Patient cohort was divided into two groups according to their weight at the time of surgery: Group 1 < 1000 g and Group 2 ≥ 1000 g. The SPSS statistical programme for Windows, version 16 (SPSS, Inc., Chicago, IL, United States of America) was used for data analysis. The Shapiro–Wilk test was used for the analysis of compliance with normal distribution. Normally distributed continuous data are presented as a mean ± standard deviation together with its ranges in brackets and nominal variables are presented as counts and/or percentages. Non-normally distributed continuous data are presented as a mean ± standard deviation and the median parameters with their interquartile ranges in brackets. For statistical comparison of group data, Student’s t-test was used for normally distributed continuous variables and Mann–Whitney U-test for non-normally distributed continuous variables. Complication and death incidences for both groups were tested for significance using the Ki-Kare ve Fisher’s exact test. The Kaplan–Meier Method was used to analyse the postoperative 30-day and 1-year survival data. All statistical tests were two-sided. A p-value of < 0.05 was considered statistically significant.
Results
General features and clinical findings of patients
We examined 103 preterm infants who underwent patent ductus arteriosus ligation through anterior mini-thoracotomy between March 2009 and November 2019. The patients were divided into two groups according to their weight at the time of surgery: Group 1 < 1000 g (n: 58) and Group 2 ≥ 1000 g (n: 45). There are some limitations of each groups. All of the patients in group 1 are extremely low birth weight babies. However, in group 2 there are 10 patients who are extremely low birth weight babies (10/45) (22%). So the patients in group 1 have the potential for more serious complications of the prematurity than the patients in group 2.
The median gestational age of the patients was 28 weeks (IQR 26–30 weeks). Fifty-nine patients (57%) were females and 44 (43%) were males. There is no statistically significant difference in terms of gender (p > 0.05). The median birth weight was 855 g (IQR 700–1050 g). On operation day, the median weight of the patients was 900 g (IQR 800–1125 g) and the median age was 21 days (IQR 14.5–29 days). In addition to these analyses, the body weights and ages on the day of operations for patients in Group 1 and Group 2 are provided in Table 1.
Table 1. General features of all cases and groups

p1: Comparison of the mean operation age for both groups; p2: Comparison of the mean operation weight for both groups; NS: Non-significant
All patients had congestive heart failure and 28 (27%) had a pulmonary infection. All patients were ventilator-dependent before surgery. In addition to patent ductus arteriosus, a preoperative echocardiographic study revealed patent foramen ovale in 39 patients (38%), ventricular septal defects in 6 patients (6%), and atrial septal defects in 11 patients (11%). Additional comorbidities were also found in patients before surgery: 17 (17%) had bacterial sepsis, 16 (16%) necrotising enterocolitis, 11 (11%) hydrocephalus and 4 (4%) intracranial haemorrhage. The youngest patient was 1 day old on operation day. The reason for this early patent ductus arteriosus closure was intracranial haemorrhage.
Surgical procedure and follow-up
The mean duration of the operation from the time of incision to the completion of skin closure was 16.26 ± 2.86 minutes (a range of 11– 29 minutes). The mean mechanical ventilatory support time after surgery was 16.17 ± 3.63 days (a range of 2–52 days), and the mean hospital stay was 64.4 ± 33.4 days (a range of 14–266 days).
In total, 12 patients (11.6%) died within the first postoperative 30 days. One of these patients whose weight was 750 g died in the operating room due to sudden cardiac arrest during the operation. Of the remaining 11 mortalities, ten were due to sepsis, and one was due to necrotising enterocolitis. In all patients, six patients (n = 6/93) (6.4%) died between 1 month and 1 year after the operation. Four patients died because of sepsis, one died after intracranial haemorrhage and one died due to bronchopulmonary dysplasia.
Six patients (5.8%) had postoperative complications (four in Group 1 and two in Group 2). Three patients (3%) required intraoperative transition to median sternotomy because of bleeding and all were repaired surgically. Residual patent ductus arteriosus was seen in one patient postoperatively. This patient was reoperated on postoperative day 4 through median sternotomy. One patient had deep venous thrombosis at his lower extremity. One patient underwent surgical revision for chylothorax and chylomediastinum causing late cardiac tamponade on postoperative day 26. No damage to the phrenic nerve or recurrent laryngeal nerve occurred.
Statistical data analyses
General data
The mean weight of all cases on operation day was 980 ± 200 g (460–1650). The mean age of all cases on operation day was 25.5 ± 20.6 days (1–172). There is a statistically significant difference in mean operation weights between Group 1 and Group 2: (800 ± 100 (460–970) g, 1200 ± 100 (1000–1650) g, respectively; p: 0.0001). Statistically, there is no significant difference in the mean operation age between two groups. (24.7 ± 17 (6–119) days, 27.4 ± 24.6 (1–172) days, respectively; p: 0.259) (Table 1).
Patient mortality and survival data analyses
The operation weight and age of patients who either died or survived in follow-up are shown in Table 2. In all cases 85 patients were survived a year after surgery. In group 1, 46 patients (46/58) (79 %); in group 2, 39 patients (39/45) (87 %) were survived. When all cases are analysed, there is no statistically significant difference in the mean operation weight and mean operation age between deceased and surviving patients (p1: 0.309 and p2: 0.517, respectively). Furthermore, there is also no statistically significant difference in the mean operation weight and mean operation age of deceased and surviving patients, when analysed in terms of Groups 1 and 2 (p3–6: 0.531, 0.700, 0.091, and 0.187, respectively).
Table 2. Features of alive and dead cases

p1: Comparison the mean operation weights of alive and dead patients in all cases; p2: comparison the mean operation ages of alive and dead patients in all cases; p3: comparison the mean operation weights of alive and dead patients in group 1; p4: comparison the mean operation weights of alive and dead patients in group 2; p5: comparison the mean operation ages of alive and dead patients in group 1; p6: comparison the mean operation ages of alive and dead patients in group 2; NS: Non-significant
When all cases are analysed, there is no statistically significant difference in mean the operation weight and mean operation age between those patients who died in the first month after surgery and those patients who survived a month after surgery (p: 0.81 and 0.49, respectively). Similarly, there is no statistically significant difference in the mean operation weight and mean operation age of patients who either died or survived a month after surgery when Groups 1 and 2 are analysed separately (p: 0.23, 0.51, 0.39, and 0.43, respectively). In addition, there is no statistically significant difference in the mean operation weight and mean operation age between patients who died in the first month and patients who died after the first month following surgery (p: 0.80, 0.92, respectively).
Complications and survival data analyses
When the complication rates of patients are compared between Groups 1 and 2, there are more cases with complications in Group 1. However, there is no statistically significant difference between the two groups (8.6 and 4.4%, respectively; p: 0.46) (Table 3). The mortality rate during the first month after surgery is 13.7% in Group 1 and 11.1% in Group 2. The mortality rate during the first year after surgery is 20.6% in Group 1 and 13.3% in Group 2. There is no statistically significant difference for either postoperative time intervals (p2: 0.68 and p3: 0.33, respectively) (Table 3).
Table 3. Short- and mid-term follow-up results

DVT: Deep Venous Thrombosis; NS: Non-significant; PT: Pericardial tamponade
P1: Comparison of the rates of complications, P2 and P3: comparison of the rates of mortality in first 30 days and 1 year, respectively
The short-term postoperative survival rates for each group at the end of day 1, day 3, day 10, and day 30 are 98 ± 1.3%, 95 ± 1.7%, 91 ± 2.6%, and 87 ± 4.3%, respectively (Fig 2). The mid-term postoperative survival rates for each group at the end of month 3, month 6 and month 12 are 82 ± 0.7%, 82 ± 1%, and 81 ± 2%, respectively (Fig 2).

Figure 2. Kaplan–Meier curves show the survival of each group in first month after surgery and a table shows the cumulative proportion of survivals in 1 year.
When the 30-day and 1-year survival rates of Groups 1 and 2 are compared, there is no statistically significant difference between the two groups (p: 0.347 and 0.347, respectively).
Discussion
After a decade and over a hundred cases under analysis, the anterior mini thoracotomy approach has become our primary surgical strategy for closure of patent ductus arteriosus in preterm infants. According to the research data for our entire patient cohort, we can speculate that this surgical procedure is particularly helpful for babies with high frequency ventilation support for whom a lateral decubitus position is not recommended. The most important advantages of this surgical approach to close patent ductus arteriosus in premature infants is that the patient is placed in the supine position with the left chest elevated for an anterior mini-thoracotomy. This surgical position is easily maintained for neonates in either the intensive care unit or in the operating room. Furthermore, this approach provides positional convenience for transition to sternotomy in emergency cases.
Patent ductus arteriosus closure with minimal invasive anterior thoracotomy is primarily derived from the technique published by Coles et al in 1963 for main pulmonary artery banding. Reference Coles, Gergely and Buttigliero5 However, there have been few studies that examine the use of this surgical approach for patent ductus arteriosus closure since then in the literature. Fouilloux et al published a study of more than 50 symptomatic preterm infants with anterior minimal invasive thoracotomy. Although, they reported one case with irreversible tearing of the main pulmonary artery, the overall research findings indicated that this surgical technique was safe and reliable. Reference Fouilloux, Gran and Kreitmann6 In another study of 21 preterm infants, Wanert et al similarly argued that surgical patent ductus arteriosus closure using anterior mini-thoracotomy was an effective technique with experienced surgeons in very low-weight preterm babies. No deaths related to surgery were reported. Reference Wanert, Louali, Ovaert and Fouilloux7
In almost five decades of treating preterm newborns for the closure of patent ductus arteriosus, the conventional surgery is lateral thoracotomy. Reference Verhaegh, Accord and Kooi8 Direct visualisation and control of the ductus minimise potential risks. However, lung injury and potential long-term spinal and chest wall deformities are also well-recognised with this surgical approach. Reference Bal, Elshershari, Celiker and Celiker9 In a study that compared posterolateral thoracotomy and median sternotomy approaches for patent ductus arteriosus closure in preterm neonates, Verhaegh et al found that the postoperative pulmonary complication rate was significantly lower in the median sternotomy patient group. Reference Verhaegh, Accord and Kooi8 These findings indicate that surgical techniques involving no touching or less traction of lung tissue is significantly valuable.
The gentle traction of lung tissue and the ribs is surgically vital in preterm newborns. In the lateral thoracotomy approach, the spreading of the ribs may contribute to postoperative ventilator dependence. Left lung compression during ligation may also cause intrapulmonary haemorrhage and atelectasis. Reference Raval, Laughon, Bose and Phillips10 In the anterior mini-thoracotomy approach, the incision is closer to the patent ductus arteriosus and therefore only the apex of the left lung is gently retracted, so the vulnerability of the lung tissue is less compared to other surgical techniques.
The two approaches to patent ductus arteriosus ligation include the conventional transpleural or the extrapleural approach. The transpleural approach may be complicated by intraoperative bleeding, pneumothorax, left vocal cord paralysis, lymphatic leak, and injury to the left phrenic nerve. Reference Demirturk, Guvener, Coskun and Tunel11 Even if premature infants do not have a surgery, they can experience a variety of complications. And the potential for complications to occur significantly increases after any surgery. In our study we focussed only on complications directly attributable to the patent ductus arteriosus operation. In our cohort, we had one patient with chylothorax and chylomediastinum on postoperative day 26. We did not routinely screen our patients by diaphragmatic scopy and broncoscopy to evaluate the functions of recurrent laryngeal nerve and phrenic nerve unless we had detected prolonged intubation or hoarseness with unknown reason. Fortunately, none of the patients had left vocal cord or diaphragmatic paralysis or paresis. This is probably due to the way that this approach provides easy surgical exposure of the left phrenic nerve and the recurrent laryngeal nerve is untouched during the patent ductus arteriosus dissection. It should also be noted that dissection of the friable patent ductus arteriosus is extremely hazardous. Therefore, using a metallic vascular clip allows for limited dissection and safe occlusion. By comparison, it has not been necessary to insert a chest tube for drainage in any of our patients with our transpleural approach, which may have improved patient comfort during the postoperative period. We have evacuated the air with a suction catheter, one end of which is attached to the underwater drainage system while simultaneously inflating the lungs before ligation of the last skin suture. The extrapleural approach has better surgical exposure and involves less surgical dissection, which results in less risk of surgical complications and haemorrhage. Reference Demirturk, Guvener, Coskun and Tunel11
The main alternatives to conventional surgery for patent ductus arteriosus closure involve video-assisted thoracoscopy and percutaneous procedures. Reference Garcia and Lukish12 While there are some potential advantages to using these techniques, vascular access and catheter manipulation during patent ductus arteriosus device closure in preterm infants can be particularly problematic for the percutaneous approach. Reference Bansal, Prabhu, Ware and Shivapuje13 Therefore, conventional surgery is generally preferred over these alternative options. Reference Stankowski, Aboul-Hassan and Seifi-Zinab14
Most of our patients (82%) have been referred from other health centers to our hospital for patent ductus arteriosus surgery and have then been transferred back just after surgery. Transferal between two health centers can adversely affect postoperative outcomes for these high-risk infant patients, including the risk of thermoregulatory instability, loss of intravenous lines, malfunction and accidental discontinuity of monitoring and improper ventilation. Reference Jennings, Innes, Wirth and Askew15 Fortunately, we have not experienced any problems due to these patient transfers.
In three patients (3%), we had to make median sternotomy because of the intraoperative massive bleeding of patent ductus arteriosus. Although we dissected the patent ductus arteriosus gently, some patent ductus arteriosus tissues were more fragile. When we were dissecting the patent ductus arteriosus or replacing the clip, unfortunately the patent ductus arteriosus was ruptured. We compressed over the patent ductus arteriosus with a sponge and immediately made median sternotomy to control the bleeding. We preferred the median sternotomy by reason of supine position of patients and with its advantage of wide and comfortable exposure of patent ductus arteriosus, heart, pulmonary artery, and aorta. The bleeding was controlled in all cases. All patients were survived after this complication.
Postoperative management of patent ductus arteriosus closure in preterm infants is a challenging problem. It requires us to be more cautious about potential problems in numerous organ systems. Indeed a few studies recorded hospital mortality rates up to 30%. Reference Raval, Laughon, Bose and Phillips10–Reference Stankowski, Aboul-Hassan and Fritzsche16 We have recorded mortality rates that are higher than most other prior studies, Reference Verhaegh, Accord and Kooi8,Reference Kang, Samsudin, Kuruvilla, Dhelaria, Kent and Kelsall19–Reference Lehenbauer, Fraser and Crawford22 (Table 4) mainly because our study involves all premature infants with patent ductus arteriosus, including babies who have severe morbidities. These mortality rates are not usually related to surgical or underlying cardiovascular pathology, but due to complications associated with prematurity. Reference Hines, Raines and Payne17 Only one of our mortality cases was due to surgical complications. This particular patient weighed only 750 g and died in the operating room due to sudden cardiac arrest after clipping the patent ductus arteriosus. The remaining 17 mortality cases died within a year of surgery, mostly due to prematurity complications and infection.
Table 4. Mortality rates of some studies in literature about surgical PDA ligation in low birth weight infants

GA: Gestational age
Our study has several limitations worth noting. Its retrospective nature and relatively small sample size limit the conclusions that may be derived. We did not attempt to compare the results of surgical ligation to medical management or other surgical approaches. Therefore, we cannot make claims about the superiority of one approach over another. Additionally, we focussed on early and mid-term morbidity and mortality in this study and did not assess patients for long-term outcomes. In terms of mid-term outcomes, the survival of our delicate patients is rarely related to the patent ductus arteriosus ligation operation itself. It is mostly related to prematurity complications in the mid- and long-term. Our intraoperative and short-term postoperative outcomes better demonstrate the success of our surgical technique for patent ductus arteriosus closure in preterm infants.
We conclude that patent ductus arteriosus ligation by anterior mini thoracotomy is a safe procedure with a low risk of surgical mortality and morbidity. Prospective randomised studies to compare anterior mini-thoracotomy to more traditional lateral thoracotomy are necessary to be conclusive on which surgical approach is most effective and safe.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
This study was approved by the Hospital Institutional Ethical Committee. Clinical registration number is 28001928-604.01.01-E.83.









