Leftward prolapse or deviation of the primary atrial septum is a CHD characterised by displacement of the superior margin of the primary atrial septum well into the left atrium, the base of the primary septum being normally formed. This results in the pulmonary venous flow to the right atrium, despite the anatomic connection of the pulmonary veins to the left atrial wall. Reference Van Praagh, Carrera, Sanders, Mayer and Van Praagh1 It thereby, depending on the degree of deviation, mimics a partial or totally anomalous pulmonary venous return. Additionally, the flap valve of the oval fossa divides the left atrium, much like as in a divided morphologically left atrium Reference Cuttone, Hadeed and Lacour-Gayet2 and a “fenestration” or a paraseptal leak is necessary to allow blood flow into the left ventricle. The differential diagnosis between these different anatomical conditions may be difficult. We present a case of a newborn infant referred for surgical correction of totally anomalous pulmonary venous return, but with the peri-operative finding of an isolated leftward prolapse of the primary septum, or was it a dividing left atrial shelf? Subsequently, embryological and anatomical features of the atrial septum are discussed. Clinical features from another 53 cases from the literature are presented.
Case report
A 6-week-old infant presented to the paediatric team with feeding difficulties and was found on examination to have discrete tachypnoea, a systolic ejection murmur on auscultation and transcutaneous oxygen saturation of 92% in room air. Initial transthoracic cardiac echography suspected totally anomalous pulmonary venous return. The baby was referred for cardiac surgery. Biometry and ECG were normal. Repeat cardiac echography (GE Healthcare Vivid E90 Ultrasound System; Fig 1) showed, in the context of situs solitus, disproportion between the left and right heart structures with, although reaching the apex, a small left ventricle. The M-mode-based left ventricle end-diastolic diameter measured 14.8 mm, corresponding to −3.7 Z-score (Heart Center Z-score calculator, Boston Children’s Hospital) and the right ventricle end-diastolic diameter 23.6 mm. The 4-chamber measurement of the mitral valve size was 9 mm (−2.1 Z-score) with no signs of dysfunction; however, the tricuspid valve measured 16.5 mm (+2.4 Z-score) with mild regurgitation. An enlarged pulmonary valve (annulus diameter of 15 mm, +2.4 Z-score) with turbulent high flow was observed, in contrast to a 6.4 mm aortic valve annulus diameter (−1.9 Z-score). The transverse aortic arch measured 7 mm (−0.37 Z-score) and the aortic isthmus diameter 4 mm (−2.0 Z-score). The pulmonary arteries measured 7 mm (+1.0 Z-score) and 6 mm (+0.2 Z-score), right and left, respectively.

Figure 1. Pre-operative transthoracic echocardiographic findings in our patient, subcostal 4-chamber views. ( a ) Bidimensional subcostal 4-chamber view showing significant leftward orientation of the primary septum and small left heart structures with a dilated RA and RV; ( b ) colour subcostal 4-chamber view showing a hypoplastic limbus superior and normal pulmonary venous connection to the left side of the atrial body; ( c ) colour subcostal 4-chamber view showing an atrial communication between the primary septum and the posterior wall of the LA permitting anterograde blood flow through the mitral valve. * = atrial septum defect; + = fenestrated displaced flap valve of the oval fossa; LA = left atrium; LS = limbus superior; LV = left ventricle; MV = mitral valve; PV = pulmonary vein; PV = pulmonary veins; RA = right atrium; RV = right ventricle.
Above both ventricles, a large atrial cavity, measuring 25 × 23 mm, was visualised, receiving systemic veins from the right side and pulmonary veins from the left side. Using colour Doppler, significant flow from the pulmonary veins was visualised, entering the cavity and reaching the right side of the cavity. Furthermore, a connection between the large atrial and a minute supra-mitral cavity, delineated by a 4 mm fenestrated membrane, was seen on colour Doppler. Restrictive and turbulent flow was evident between the large and smaller cavities. At first sight, the large atrial cavity was interpreted as an enlarged right atrium and the small supra-mitral cavity as left atrium, with pulmonary flow above the left atrium towards the right atrium and thus totally anomalous pulmonary venous return.
On closer inspection, however, the superior interatrial fold seemed hypoplastic with a very small rim superiorly in the middle of the larger atrial cavity. The membrane between the atrial cavities represented to us the primary septum, commonly called the flap valve of the oval fossa, shifted leftwards and attached on the posterior left atrial wall, creating an image of divided left atrium. No additional imaging was performed.
During surgical inspection, there was the impression of a mono-atrium, with drainage of all four pulmonary veins into the left wall of the large atrial cavity. The superior part of the primary septum was indeed visualised as a membrane right above the mitral valve, dividing the left atrium as in a divided morphologically left atrium or so-called “cor triatriatum sinister.” This partition divided the left atrium sub-totally with minimal shunt laterally and through a fenestration. After inspection, the partition was detached and mobilised towards its normal position and fixated by a simple suture to the small infolded superior rim in the atrial roof. Fenestration was closed by a single suture. Given the normal anatomical drainage of all four pulmonary veins, no rerouting was performed. The post-operative course was uncomplicated. Cardiac ultrasound confirmed complete closure of the interatrial septum, normal pulmonary venous return into the left atrium and unobstructed left ventricle inflow. Further follow-up was uneventful.
Discussion
Clinically significant leftward prolapse of the primary septum was described by Van Praagh et al. Reference Van Praagh, Carrera, Sanders, Mayer and Van Praagh1 Usually, it is associated with hypoplastic left heart syndrome or in isomerism syndromes. Reference Van Praagh, Carrera, Sanders, Mayer and Van Praagh1,Reference Chin, Weinberg and Barber3–Reference Silvestri, Scarabotti and Marino5 Few cases of isolated forms in young infants such as our patient have been described. Understanding the embryology of the normal atrial septum development as well as reviewing the case studies existing of this malformation assist in the understanding of this anatomic abnormality.
Embryological features of atrial septation
Firstly, in reviewing the morphogenenis of leftward prolapse of the primary septum, an appropriate account of the embryology of atrial septation should be discussed. Atrial septal development is a complicated topic and largely described in the literature. Based on Anderson’s work, Reference Anderson, Brown, Mohun, Wernovsky, Anderson and Krishna6 the theory that the secondary atrial septum arises from the atrial roof and overlaps the primary septum is inaccurate. Indeed, the septation of the atrial component of the heart initiates from a ridge of mesenchyme in the roof of the undivided atrial cavity. We first see the appearance of the primary atrial septum, which grows as an interatrial shelf. At that time, endocardial cushions have developed within the atrioventricular canal. These cushions grow towards each other to divide the canal, whilst the primary septum grows towards the cushions. By this movement, the cranial portion of the septum, at its origin from the atrial roof, has broken down, creating the secondary interatrial foramen. This hole is an essential part of the foetal circulation, permitting the placental oxygenated rich blood to return and reach the left side of the developing systemic circulation. Once the pulmonary veins are incorporated into the atrial roof, the upper margin of this foramen become converted into the interatrial fold. The superior rim of the oval fossa, better described as the superior interatrial fold, is thus a false septum, although commonly described as the secondary atrial septum.
A potential ambiguity in the terminology currently employed by Anderson exists at this stage. The superior fold can be interpreted as representing a “buttress” against which the flap valve will abut so as to close the oval fossa. Nonetheless, the current account of septal development endorsed by Jensen et al Reference Jensen, Spicer, Sheppard and Anderson7 emphasises that there is a true second atrial septum involved in the atrial septation. The second atrial septum originates from the vestibular spine and then muscularises antero-inferiorly to form the buttress for the flap valve of the definitive oval fossa, the flap itself being formed by the primary atrial septum. This version leaves the superior interatrial fold as a superior buttress and the second septum as an antero-inferior one. After birth, the pulmonary venous drainage into the left atrium dramatically increases, and the right aspect of the primary septum, represented by the flap valve of the oval fossa, is pushed against (and must fuse with) the left aspect of the superior interatrial fold, closing the oval fossa. The true second atrial septum anchors the flap valve to the atrioventricular junctions.
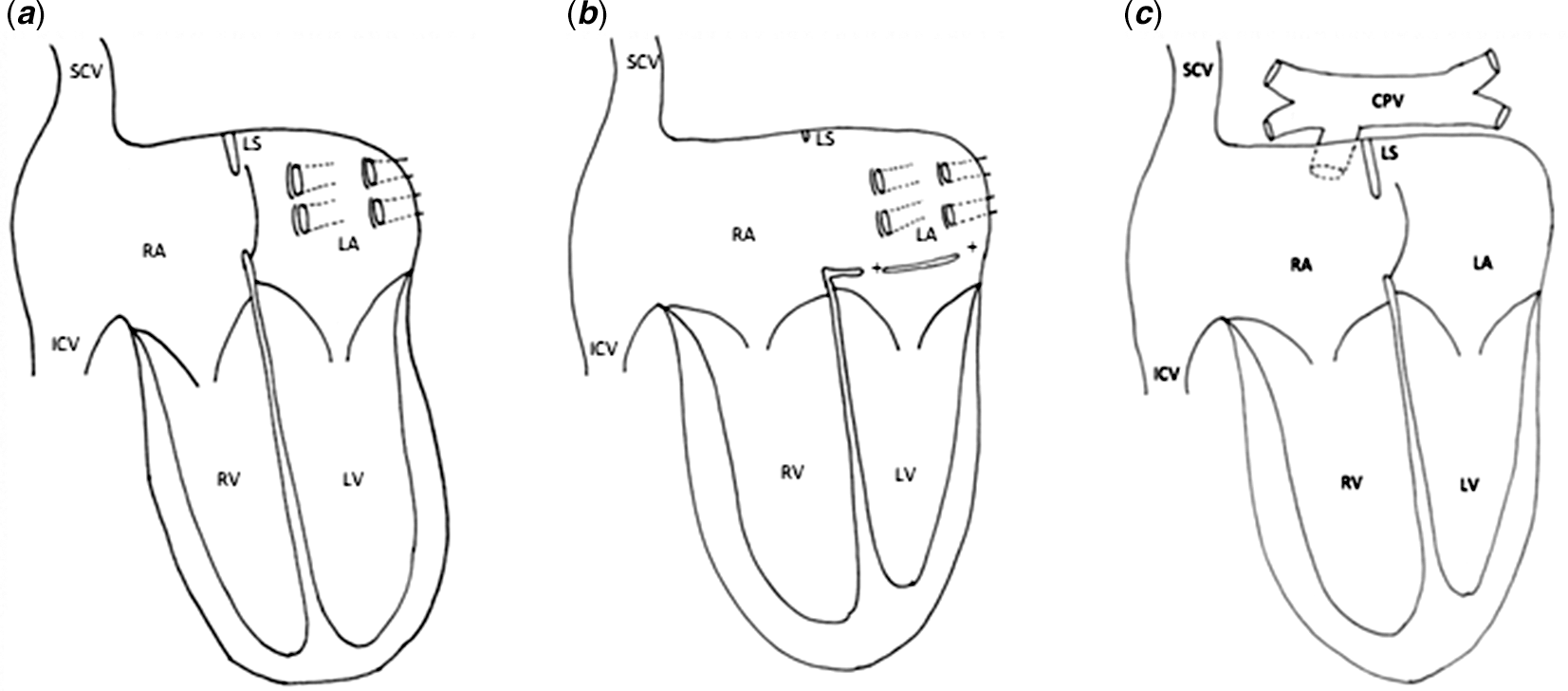
Van Praagh’s description of the development of the atrial septum is different. Reference Van Praagh, Carrera, Sanders, Mayer and Van Praagh1,Reference Cuttone, Hadeed and Lacour-Gayet2 The infolding of the atrial roof results in a superior limbic band, being part of the secondary atrial septum. It is against this limbic band that the cephalic border of the primary septum normally attaches. However, to our knowledge, there is no evidence from developing hearts that a second septum grows from the atrial roof. Anyway, normal development of the superior folding is essential for the atrial septation. If the superior limbic band is hypoplastic, resulting from an insufficient infolding of the atrial roof, it provides insufficient support to the superior border of the primary septum allowing prenatal prolapse towards the left atrium driven by the foetal blood flow. Reference Van Praagh, Carrera, Sanders, Mayer and Van Praagh1 If the flap valve of the primary septum deviates beyond the pulmonary veins, postnatal increase of the pulmonary venous return will not push the primary septum against the superior interatrial fold and will not close the oval fossa (Fig 2a and b).

Figure 2. Schematic presentation of cardiac anatomy in differential diagnosis, ventricular outflow tracts and great vessels not represented. ( a ) Normal cardiac anatomy at birth; ( b ) leftward prolapse of the primary septum, ( c ) intracardiac totally anomalous pulmonary vein return. + = fenestrated displaced flap valve of the oval fossa; CPV = common pulmonary vein; ICV = inferior caval vein; LA = left atrium; LS = limbus superior; LV = left ventricle; RA = right atrium; RV = right ventricle; SCV = superior caval vein.
Clinical features
Secondly, we conducted a systematic search to identify all cases published with deviation of the primary septum causing abnormal pulmonary venous flow. The following search terms were used to search for relevant articles in PubMed: (“Atrial Septum”[Mesh] OR atrial-septum [tiab] OR septum-primum [tiab]) AND (Malposition [tiab] OR displacement [tiab] OR attachment [tiab] OR deviation [tiab] OR prolapse* [tiab]). All English articles describing one or more cases of deviation of the primary septum causing abnormal pulmonary venous return were suitable for inclusion. Articles were only included if the full text was available online. The following data were extracted from the articles: author(s), year of publication, age of the patient(s), presenting symptoms, situs, aspect of the superior, partial or total abnormal pulmonary venous drainage, associated cardiac pathology, treatment and outcome. In total, 126 articles were identified. Based on title and abstract, 110 articles were excluded. The full text of 16 articles was available for review and 8 articles were suitable for inclusion. In total, 53 cases were described in detail. An overview of these cases is given in Table 1. From this analysis, we concluded that age at diagnosis varied between 0,3 and 120 months. Potential symptoms at presentation varied with showing signs of cardiac failure and other being asymptomatic. Common features mentioned are transcutaneous desaturation, dyspnoea, arrhythmia and heart murmurs.
Table 1. Published clinical cases with deviation of the septum primum causing abnormal pulmonary venous return (or drainage).

ASD = atrial septal defect; AVSD = atrioventricular septal defect; BSVCV = bilateral superior vena caval veins; DASP = deviation of the septum primum; DORV = double outlet right ventricle; IVC = inferior vena cava; LAI = left atrial isomerism; LLPV = left lower pulmonary vein; LPSVC = left persistent superior caval vein; LUPV = left upper pulmonary vein; LV = left ventricle; PAPVD = partial pulmonary venous drainage; PDA = patent ductus arteriosus; RAI = right atrial isomerism; RLPV = right lower pulmonary vein; RUPV = right upper pulmonary vein; TAPVD = total abnormal pulmonary venous drainage; TTE = trans thoracic echocardiography; VSD = ventricular septal defect.
*with azygos continuation.
+Three out of five cases were diagnosed using TTE. Two out of five cases were diagnosed using catherisation.
The diagnosis can be confirmed using two-dimensional transthoracic cardiac echography, with following features being key signs for the diagnosis: leftward displacement of the primary septum, dividing the left atrium, thereby allowing half or all of the pulmonary veins to drain into the right atrium, despite their anatomic connection to the left atrial wall; hypoplastic or absent infolded superior rim; small appearance of the left ventricle and left atrium beneath the flap; enlargement of the right atrium and ventricle; secondary interatrial communication.
Van Praagh called it a “primary septum malposition defect” as it presents an interatrial communication associated with the (partial) absence of the secondary atrial septum and malposition of primary septum. Reference Van Praagh, Carrera, Sanders, Mayer and Van Praagh1,Reference Cuttone, Hadeed and Lacour-Gayet2 In fact, in divided left atrium, the interatrial communication can be open either to the pulmonary or the vestibular component of the divided atrium. In either event, however, it is the secondary interatrial communication. This is because the communication is between the cranial edge of the primary atrial septum, or the flap valve, and the roof of the atrial chambers. The position of the communication does not change its developmental heritage. It is the anatomical borders that determine the phenotypic specificity. One or more fenestrations in the primary septum can coexist, as in our case.
It is clear from an echocardiographic point of view, leftward prolapse of the primary septum may be misdiagnosed. Reference Cuttone, Hadeed and Lacour-Gayet2,Reference Cohen, Weinberg, Coon, Gaynor and Rychik17 The images can be similar to intracardiac partial or totally anomalous pulmonary venous return into the right atrium. In our case, because all four pulmonary veins drained into the left-sided atrial wall, this condition could not be considered as totally anomalous pulmonary venous return, even if the drainage is above the primary septum and in terms of physiology, similar to totally anomalous pulmonary venous return (Fig 2c). Moreover, it is also exceedingly rare to find totally anomalous pulmonary venous connection directly to the morphologically right atrium other than in the setting of right isomerism.
Regarding the entity of divided left atrium, it is defined by an obliquely orientated fibromuscular partition dividing the morphologically left atrium. Reference Anderson, Spicer, Redington, Wernovsky, Anderson and Krishna9 There are many classifications described with high variability in the position of the pulmonary veins and the position of the communication between the compartments. The morphogenesis of the divided left atrium is conventionally explained on the basis of failure of absorption of the common pulmonary vein into the left atrium. Another hypothesis is the “entrapment concept” of the primary pulmonary vein, in combination with “malseptation,” if the atrial septum defect communicates with the proximal chamber. Reference Anderson, Spicer, Redington, Wernovsky, Anderson and Krishna9 Cuttone et al believe that the “primum movens” of leftward prolapse of the primary septum is different, with failure of the development of the superior limbic band of the secondary atrial septum, insisting on a different position of the atrial septal defect, in a low position near the atrioventricular junction in the classical form of divided left atrium. In leftward displacement of the primary septum, the communication is higher, far from the atrioventricular junction. Reference Cuttone, Hadeed and Lacour-Gayet2 These theories, on differentiating divided left atrium from leftward prolapse of the primary septum, are speculative. None of the previous investigators have provided any evidence to prove the true nature of the partition. In our clinical case as in most of the reviewed literature cases (Table 1), there is no histologically evidence neither to confirm that the partition dividing the cavity of the left atrium is the deviated primary atrial septum, and not a dividing left atrial shelf as in the setting of a divided morphologically left atrium. In future cases, if it proved possible to remove the partition and study it histologically, Reference Van Praagh and Corsini10 it should be possible to determine whether it is the primary septum or a dividing shelf.
Correct diagnosis however facilitates surgical management. When available and feasible, pre-operative 3D-transthoracic echocardiography is a valuable tool to visualise the intracardiac anatomy and to determine the operative strategy. Reference Cuttone, Hadeed and Lacour-Gayet2 The most frequently used surgical technique to correct a leftward prolapse of the primary septum consists of a complete resection of the malpositioned flap valve followed by the reconstruction of an appropriately positioned septum using either pericardium or prosthetic material. Reference Van Praagh, Carrera, Sanders, Mayer and Van Praagh1 It is important to perform a complete resection of the primary septum to prevent pulmonary venous obstruction. Reference Hiramatsu, Takanashi and Imai11,Reference Turley, Tarnoff, Snider and Ebert12 Another technique, namely performing a septoplasty using the original primary septum, has been described to avoid patch retraction and to allow natural septal growth. Reference Van Praagh, Carrera, Sanders, Mayer and Van Praagh1,Reference Hiramatsu, Takanashi and Imai11 Otherwise, as re-siting the primary septum, repositioning of a dividing shelf is also feasible. This second technique was used with success in our case. This technique is however often not applicable as the partition (dividing shelf or primary septum) may be severely displaced and malformed or adherent to the left atrial wall. Reference Cuttone, Hadeed and Lacour-Gayet2 Prognosis of isolated forms of leftward prolapse of the primary septum is expected to be very good as no pulmonary venous rerouting is necessary.
Conclusion
Leftward prolapse of the primary septum is considered as the deviation of the superior margin of the primary atrial septum, dividing the left atrium. Pulmonary veins are externally normally connected to the left atrial wall, but the drainage of pulmonary venous flow visually goes into the right atrium. This may be incorrectly diagnosed as partial or totally anomalous pulmonary venous return. Moreover, there is currently no evidence to substantiate that the described partition is initially a deviated primary septum rather than a dividing atrial shelf. The existing theories for the development of the atrial septum are not able to answer the question about the true nature of the partition in this type of divided left atrium. Correct diagnosis, however, is important to facilitate surgical repair and to predict prognosis.
Acknowledgement
The authors thank Dr K. Carkeek of University Hospital Saint-Luc, Brussels, for proofreading the manuscript.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.