GrantReference Grant1 first described abnormal left ventricular morphology with increased trabeculation and a meshwork of blood-filled, endocardially lined spaces from an autopsy on a 14-month-old female child with single ventricle heart disease. The inter-trabecular recesses were in effect, myocardial sinusoids, and therefore likely distinct from the contemporary definition of left ventricular non-compaction. Subsequent definitions by Jenni et alReference Jenni, Oechslin, Schneider, Jost and Kaufmann2 were based on seven patients with left ventricular non-compaction and no associated cardiac anomaly, in comparison with <10 patients with hypertensive heart disease and dilated cardiomyopathy. These authors thus excluded any confounding haemodynamic load imposed by associated CHD. FinstererReference Finsterer3 observed a high prevalence of associated cardiac disease in left ventricular non-compaction patients; however, none of these patients had complex CHD.
Case series reviewing non-compaction in CHD demonstrate higher ventricular end-diastolic volumes without significant changes in ventricular performance, although these have not included patients with complex CHD.Reference Madan, Mandal and Bost4 The largest case series by Stahli et alReference Stahli, Gebhard and Biaggi5 identified 24 patients with left ventricular non-compaction, of whom 3 had complex CHD but the denominator was not known. The observation of extensive trabeculation in “single ventricle” heart disease is only obtainable from case reports.Reference Lauer, Fink, Petry, Dunn and Diehl6–Reference Kim, Park and Lee8
The association between single ventricle heart disease and non-compaction is thus rarely reported, which is unsurprising considering the low prevalence of each of these conditions, but another reason may be due to associated CHD having often been excluded from the definition of non-compaction. The implications of abnormal loading conditions on ventricular trabeculation are unknown. Prompted by our experience of the prevalence of left ventricular non-compaction in our adult patients, we sought to review its prevalence in adults with single ventricle heart disease and to investigate its distribution, as well as potential effects on ventricular performance.
Methods
Study population
Imaging data from patients aged ≥15 years with single ventricle heart disease undergoing cardiac MRI between 2000 and 2014 at three tertiary centres were retrospectively reviewed. Patients deemed suitable for 1.5 ventricular repairs or those with a reasonable sized functional second ventricle were excluded. Clinical data, including type of palliative shunts or procedures, presence of arrhythmia or embolic complications, were obtained from medical records.
Ethics approval was obtained from each participating institution. As this was a retrospective study reviewing cardiac MRI scans that had been performed for patients as part of clinical management, the relevant Ethics Committees waived the requirement for individual patient consent.
Cardiac MRI protocol
Cardiac MRI was performed for clinical indications, and images were analysed retrospectively. Cardiac MRI was performed on 1.5T scanners (MAGNETOM Aera, Siemens Healthcare GmbH; Ingenia, Philips Healthcare; Signa Twinspeed, GE Healthcare) using phased-array receiver coils during suspended respiration. Balanced steady-state free precession cine images were acquired in vertical long-axis (two chamber), horizontal long-axis (four chamber), and short-axis planes using retrospective electrocardiographic gating (image parameters: slice thickness = 8 mm; in-plane spatial resolution 1–1.3 mm2; and temporal resolution = 40 ms).
Combined ventricular volumes of the dominant ventricle and the rudimentary second ventricle were obtained. Assessments of left ventricular volumes were performed by manual segmentation of short-axis cine images with endocardial outline at end-diastole and end-systole (Circle cvi42, Calgary, Canada; OsiriX, Bernex, Switzerland; Medis, Leiden, Netherlands; based on institution). Simpson’s rule was used to calculate the ejection fraction from these volumes. Mass was calculated by subtraction of the end-diastolic volume from the total epicardial mass at end-diastole, and the derived volume was converted to mass by multiplication with the myocardial density constant 1.05.
Cardiac stroke volumes were quantified in patients by subtracting end-systolic from end-diastolic volumes in patients. Cardiac output was derived as a product of heart rate and stroke volume. Cardiac volumes and masses were indexed to the body surface area.
Left ventricular non-compaction was diagnosed using the most widely utilised Petersen’s cardiac MRI criteriaReference Petersen, Selvanayagam and Wiesmann9 of non-compacted:compacted ratio of >2.3 in the worst affected segment in one long-axis view (Fig 1). Non-compacted:compacted ratio was measured in each of the three long-axis views, and the highest ratio was recorded. “Abnormal compaction” was defined as the presence of a bilayered structure with a thickened trabeculated layer that did not meet Petersen’s criteria for non-compaction (those that would have been otherwise classified as “hypertrabeculated”). An arbitrary cutoff of non-compacted:compacted ratio of 1.5–2.3 was specified to classify these patients.
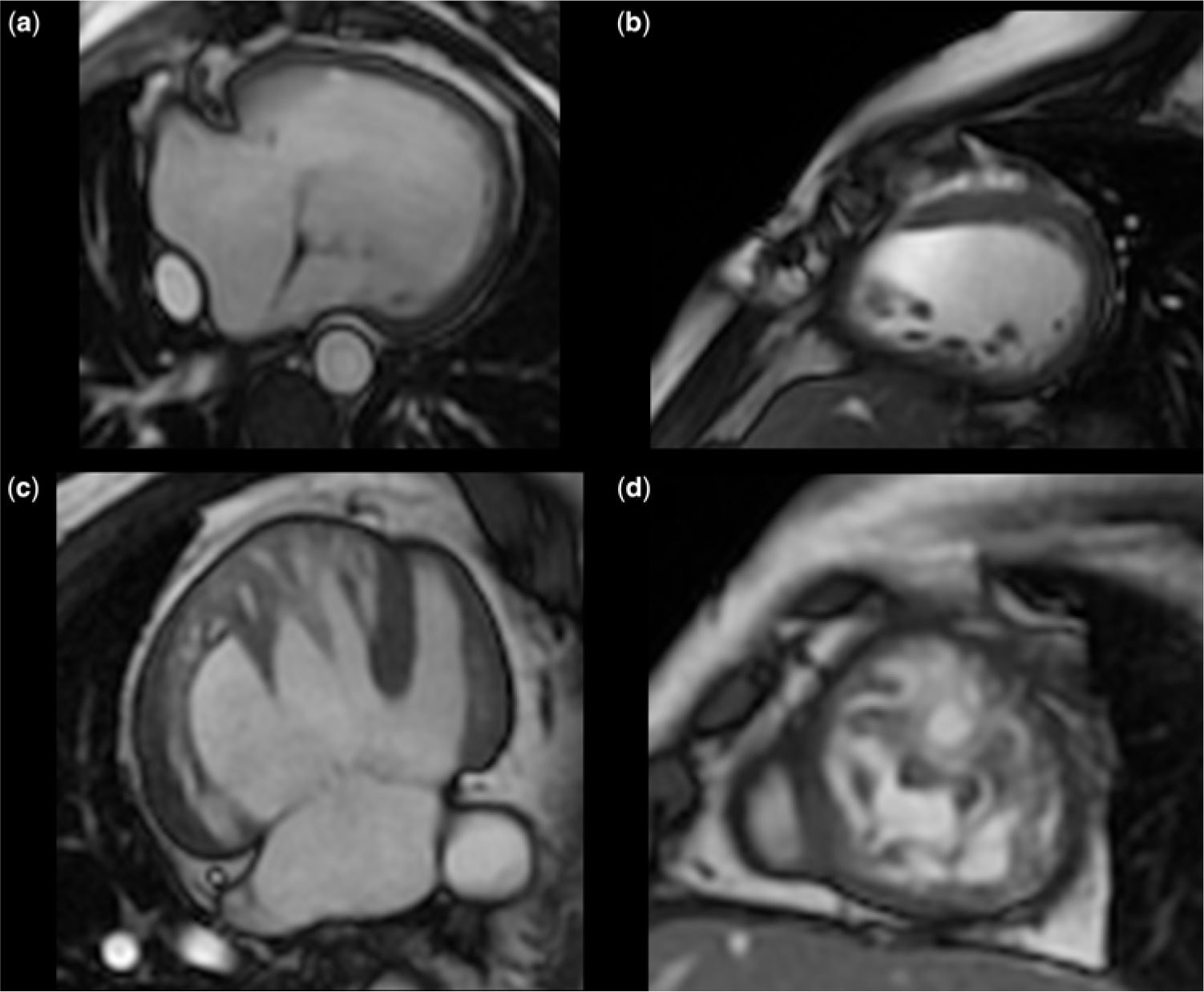
Figure 1. Cardiac MRI images of single ventricle hearts: (a) ventricular long-axis view and (b) short-axis view in a normally compacted left ventricular morphology single ventricle heart. Left ventricular non-compaction in single ventricle heart in (c) ventricular long-axis view and (d) short-axis view.
Ventricular volumes and stroke volumes were indexed to body surface area. Image processing was performed offline using the relevant workstation (Siemen’s systems or Osirix platform).
Ventricular morphology was assessed based on the ventricular inflow valve, presence of the moderator band, and extent of trabeculation. In one patient with right atrial isomerism, the ventricular morphology was indeterminate.
Statistical analysis
Continuous variables were assessed with independent samples t-tests when the data were distributed normally and categorical variables were compared using Chi-square tests. Data with non-normal distribution were compared using non-parametric tests. Bivariate correlations between continuous variables were performed using Pearson’s correlation methods. Analysis of variance was used to compare three groups of patients where there was a Gaussian distribution of data. Where the data were not normally distributed, a non-parametric Kruskall–Wallis test was performed. A two-tailed value of p < 0.05 was considered significant. Statistical analysis was performed using SPSS software (Version 22; IBM, Armonk, New York, United States of America).
Results
Patient characteristics
Of 93 patients with single ventricle heart disease, 65 had left ventricular morphology and are the subject of this report. Mean age was 24 ± 8 years (range 15–39 years). Fifty (55%) of the recruited patients were male. Two patients with abnormal compaction had protein-losing enteropathy. Systemic thromboembolism had occurred in two patients. Patient characteristics are detailed in Table 1.
Table 1. Patient Characteristics and Ventricular morphology

* otherwise unspecified
Ventricular volumes
There were no significant differences in indexed ventricular volumes, stroke volumes, or ejection fraction between patients with a morphological left ventricle versus morphological right ventricle. Figure 2 shows a comparison of ventricular volumes and function based on morphological ventricular type. Increasing age was modestly correlated with increasing left ventricular end-diastolic volumes (r = 0.34, p = 0.007).

Figure 2. Comparison of ventricular volumetric data in patients with left ventricular morphology single ventricle hearts with those with right ventricular morphology (NS = not significant).
Left ventricle non-compaction in left ventricle-type single ventricle heart disease
Left ventricular non-compaction was present in 24 patients (37%), while extensive trabeculation that did not meet criteria for non-compaction was present in another 18 patients (hypertrabeculation); only 23 (35%) had normal compaction. The mean non-compacted:compacted ratio in patients with left ventricular non-compaction was 2.94 ± 0.6, while the ratios for those with hypertrabeculation were significantly lower at 2.22 ± 0.5 (p < 0.001). A mean of 4 ± 2 segments was affected.
Distribution of non-compaction
A total of 408 myocardial segments of 24 patients with non-compaction were analysed. Overall, 103 segments (25%) met the criteria for left ventricular non-compaction, including 71 apical segments, 13 mid-ventricular, and 3 basal segments. The apex was universally involved in all non-compaction patients, mid-ventricular involvement was present in seven patients, and the basal segments were affected in four patients.
Non-compaction most frequently involved the lateral wall (21 patients, 22 segments) followed by the inferior (19 patients) and anterior walls (14 patients, 16 segments). Septal involvement was infrequent and noted in four patients. The distribution is shown in Figure 3.

Figure 3. Distribution of left ventricular non-compaction: number of patients with non-compaction in the segment shown below. Number of patients with involved segments. Red: >15 patients. Orange: 10–15. Yellow: 5–10. Green: <5.
The maximum non-compacted:compacted ratio was strongly associated with greater number of ventricular segments involved (r = 0.847, p < 0.001), with a higher number of mid-ventricular segments involved (r = 0.55, p = 0.007). Maximal non-compacted:compacted ratio did not correlate with age, ejection fraction, or ventricular volumes. The number of segments involved also correlated modestly with stroke volume (r = 0.338, p = 0.008), but no significant relationship with ventricular function was observed.
Effect of non-compaction on ventricular volumes and function
Left ventricular non-compaction patients had significantly higher indexed end-diastolic (128 ± 44 versus 104 ± 46 mL/m2, p = 0.047) and end-systolic left ventricular volumes (74 ± 35 versus 56 ± 35 mL/m2, p = 0.039) compared to those without left ventricular non-compaction. Ejection fraction was significantly lower in patients with non-compaction (44 ± 11 versus 50 ± 9%, p = 0.039). No significant differences in stroke volumes (65.0 ± 29 versus 73 ± 37 mL/m2, p = 0.36) were observed. Figure 4 shows a comparison of left ventricular volumes and function in patients with non-compaction compared to those with normal compaction.

Figure 4. Ventricular volumes and function versus left ventricular non-compaction status.
Effect of “abnormal” compaction
Eighteen patients had a moderately elevated non-compacted:compacted ratios that did not meet Petersen’s criteria for left ventricular non-compaction but still had a non-compacted:compacted ratio >1.5 and were thus arbitrarily defined as having “abnormal compaction”.
Comparing the three groups, there were no significant differences in indexed left ventricular end-diastolic (p = 0.07) or end-systolic volumes (p = 0.09). Ejection fraction did not significantly differ between the three groups (p = 0.22). Patients with abnormal compaction had a significantly higher stroke volume compared to those with normal compaction (p = 0.003) (Fig 5).

Figure 5. Effect of abnormal compaction on ventricular volumes and function.
Discussion
To our knowledge, this is by far the largest study to date concerning left ventricular non-compaction in patients with single ventricle heart disease. Three important concepts are highlighted. Firstly, left ventricular non-compaction is frequent in patients with single ventricle heart disease with a morphologic left ventricle, affecting over a third (37%) of these patients. Secondly, ventricular involvement is not extensive, but rather localised to the apex and mid-ventricle in the majority of cases. Finally, the presence of non-compaction was associated with increased ventricular volumes and reduced ventricular ejection fraction.
The interaction between abnormal haemodynamic loading conditions associated with CHD and the presence of left ventricular non-compaction is poorly understood. In a paediatric cohort of Ebstein’s anomaly patients, non-compaction was associated with higher risk of adverse events including increased mortality and progressive left ventricular dysfunction.Reference Pignatelli, Texter and Denfield10 Stahli et alReference Stahli, Gebhard and Biaggi5 speculated that haemodynamic influences are important in the pathogenesis of non-compaction, in their series of 24 patients with non-compaction and associated CHD. However, none of these patients had single ventricle heart disease and apart from rare case reports,Reference Miyahara, Kasahara, Ishino, Sakurai and Sano11,Reference Shimada, Sakamoto, Umezu and Harada12 the association of single ventricle heart disease with non-compaction as well as its effect on clinical outcomes has not been assessed in large patient series. This is because both these conditions are relatively uncommon; the reported prevalence of left ventricular non-compaction ranges from 0.014 to 0.20%, while the prevalence of single ventricle heart disease in adulthood is 0.05 per 1000.Reference Marelli, Ionescu-Ittu, Mackie, Guo, Dendukuri and Kaouache13 The number of adults with single ventricle heart disease is likely to increase by approximately 60% by 2023, requiring increasing levels of care, as the predicted prevalence of patients in NYHA Class III will double.Reference Coats, O’Connor, Wren and O’Sullivan14 Understanding the mechanisms of left ventricular non-compaction in this group is thus particularly important. The incorporation of cardiac MRI as the imaging modality of choice in adult single ventricle heart diseaseReference Baumgartner, Bonhoeffer and De Groot15 will likely reveal non-compaction more frequently. It is interesting to note that the prevalence was not particularly related to the underlying CHD lesion.
The distribution of non-compaction in single ventricle heart disease is similar to that noted in isolated non-compaction and classically involves the apical segments with lateral or inferior involvement.Reference Jenni, Oechslin, Schneider, Jost and Kaufmann2,Reference Chin, Perloff, Williams, Jue and Mohrmann16 On average, only 4 ± 2 myocardial segments were involved in left ventricular non-compaction hearts, compared to more extensive involvement in isolated left ventricular non-compaction.Reference Stacey, Andersen, Clair, Hundley and Thohan17 Most of the current diagnostic criteria have excluded patients with associated CHD due to complex haemodynamic loading conditions. Lilje et al demonstrated a similar prevalence of cardiomyopathy, arrhythmia, and thromboembolic risks in patients with associated CHD versus those with isolated left ventricular non-compaction.Reference Lilje, Rázek and Joyce18 Although mortality was higher in the CHD group, the differences were not statistically significant.
Patients with single ventricle heart disease require complex clinical management and are increasingly being referred for cardiac MRI for assessment of Fontan pathways and ventricular function. The significance of excess myocardial trabeculation incidentally noted on cardiac MRI is poorly understood. Zemrak et alReference Zemrak, Ahlman and Captur19 found no significant effects of non-compaction diagnosed on cardiac MRI by Petersen’s criteria on ventricular volumes, systolic function, or mortality over a 10-year period. Although assessing a patient’s pre-test probability of having left ventricular non-compaction is an important consideration when applying these criteria, the prevalence of left ventricular non-compaction in single ventricle heart disease remains unknown and most left ventricular non-compaction cohort studies exclude patients with CHD.
Hughes et alReference Hughes, Carstensen, Wilkinson and Weintraub20 demonstrated a high prevalence of univentricular physiology in an angiography-based diagnosis of left ventricular non-compaction in a paediatric cohort of patients with CHD. The presence of both left ventricular non-compaction and single ventricle heart disease increased the cumulative risk of adverse events by 4.7. These discrepant findings may be related to either the patient population (single ventricle heart disease patients) or the imaging modality used (cardiac MRI versus angiography). Our data demonstrate a high prevalence of left ventricular non-compaction in patients with single ventricle heart disease using Petersen’s criteria for cardiac MRI, supporting the findings of previous studies.
Furthermore, our finding of the association between left ventricular non-compaction and reduced systolic function has important clinical implications. In cohort studies of isolated non-compaction, impaired systolic function is the most consistent prognostic risk factor for adverse events.Reference Brescia, Rossano and Pignatelli21 Although patients with single ventricle heart disease are closely followed up in specialist centres, awareness of the impact of left ventricular non-compaction on ventricular function would be important in assessing the frequency of clinical follow-up, screening for symptoms of heart failure and impaired exercise capacity, and may lead to more frequent quantitative assessment of systolic function with cardiac MRI. Awareness of the associated complications of non-compaction such as arrhythmia and thromboembolic disease would also be important considerations in clinical management. However, longitudinal studies are required to assess the prognostic significance of non-compaction in this cohort.
The effect of qualitatively increased trabeculation is noteworthy. Even at an intermediate non-compacted:compacted ratio that did not meet criteria for non-compaction, we noted increased indexed stroke volumes without any significant differences in ventricular volume or ejection fraction. We hypothesise that this may be related to an adaptive, compensatory response to increased volume load. As maximal non-compacted: compacted thickness crossed the left ventricular non-compaction diagnostic threshold, the stroke volume decreased in concert with ejection fraction, which may be suggestive of a threshold of maladaptive remodeling. The lack of a direct correlation between the extent of non-compaction and ventricular volumes may be a reflection of the limitations of current diagnostic criteria that rely on a single dimension as opposed to a more comprehensive “whole ventricle” quantification method. Exercise testing would be beneficial in assessing the presence of specific adaptive effects of a small extent of trabeculation.
Limitations
Our sample size is relatively small, although substantive for a study of left ventricular non-compaction with single ventricle heart disease. The study design is retrospective. Another limitation is that data regarding arrhythmia were not available for all patients. Ventricular arrhythmia in single ventricle heart disease is multi-factorial. The presence of an implanted device would have precluded patients from cardiac MRI. Prospective assessment of electrical abnormalities would assist in determining whether non-compaction is a significant contributing factor and could inform long-term management. Only a very small subset of patients in this series underwent formal cardiopulmonary exercise testing, which precluded meaningful assessment of associations with maximal oxygen consumption in non-compaction patients. A larger study with more uniform cardiopulmonary exercise testing is required to assess this association further and could yield valuable insights into functional limitations associated with left ventricular non-compaction.
Conclusion
In patients with single ventricle heart disease of the left ventricle type, we demonstrate that left ventricular non-compaction has a high prevalence regardless of the underlying lesion and is associated with impaired ventricular systolic function. This adds to existing evidence that haemodynamic influences are likely important in the pathogenesis of non-compaction and that having left ventricular non-compaction may lead to functional consequences.
Financial Support
Dr Choudhary was funded by a NHMRC and National Heart Foundation Co-funded post-graduate research scholarship (#1055773) for part of the duration of this study.
Conflicts of Interest
None.
Ethical Standards
‘The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant Australian national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees at the Royal Prince Alfred Hospital, The Prince Charles Hospital, and University of Nebraska Medical Centre.








