Multifocal atrial tachycardia is a rare tachycardia typically observed in children under 6 months of age and can be associated with ventricular dysfunction. The mechanism of multifocal atrial tachycardia is unknown but has electrocardiographic features similar to atrial fibrillation. Similar to the heterogeneity surrounding atrial fibrillation initiation and propagation, there has been some speculation that the pathophysiology of multifocal atrial tachycardia involves a single localised atrial focus from which activation propagates and breaks down into fibrillatory conduction in the rest of the atria. There is no standard singular anti-arrhythmic agent that is used for multifocal atrial tachycardia, and treatment generally consists of atrioventricular nodal blocking agents for rate control. Ivabradine has been successfully utilised in children to treat junctional ectopic tachycardia and ectopic atrial arrhythmias and has successfully converted an adult with paroxysmal atrial fibrillation to sinus rhythm. This is the first report of using ivabradine, a selective inhibitor of the funny current (If), to organise a multifocal atrial tachycardia into a single ectopic atrial tachycardia.
Case report
A 5-month-old full-term 7.1-kg Caucasian male presented to the emergency room with a 1-week history of irritability and poor feeding. On examination, he was irritable, tachycardic, and tachypneic with decreased pulses and peripheral perfusion. A 12-lead electrocardiogram (Fig 1) showed a predominantly rapid narrow complex tachycardia with variable ventricular conduction and multiple different P wave morphologies consistent with either atrial fibrillation or multifocal atrial tachycardia. The child was in extremis with an initial pH of 7.1. Initial laboratory studies revealed a normal sedimentation rate and C-reactive protein, likely suggesting this was not myocarditis. The initial B-natriuretic peptide was 4507 pg/ml (normal 0–100 pg/ml). An echocardiogram showed a dilated left ventricle (left ventricular (LV)) end-diastolic dimension 35-mm Z-score 5.24) with an ejection fraction of 12%. The child was sedated and intubated. Cardioversion was attempted, up to 2 J per kg, with no effect, likely ruling out atrial fibrillation. The patient was placed on an esmolol infusion (100 mcg/kg/minute) and received a single bolus (2.5 mg/kg) of intravenous amiodarone over 60 minutes and placed on a continuous intravenous amiodarone infusion at 5 µg/kg/minute. Another attempt with direct current (DC) cardioversion at 2 J/kg failed to terminate the tachycardia. Over the course of 2 hours, the pH normalised and the renal near-infrared spectroscopy was depressed to 40% despite low-dose milrinone. The arrhythmia remained unchanged with occasional non-sustained ventricular rates approaching 280 bpm with the tachycardia in a 1:1 atrial–ventricular relationship. Given the low cardiac output, the patient was proactively placed on full-support venoarterial (VA) extracorporeal membrane oxygenation (ECMO). Over the next few hours, the patient was stable on full-support ECMO but with poor arterial pulsatility and ventricular rates remaining between 180 and 270 bpm despite two additional boluses of amiodarone. The following morning with the clinical picture relatively unchanged, and ivabradine was administered via the nasogastric route at 0.18 mg nasogastric twice daily (0.05 mg/kg/day). Within 6 hours of receiving the second nasogastric dose of ivabradine, the tachycardia slowed and the prior electrocardiogram which showed multiple different P waves appeared to have a singular P wave focus (Fig 2). The patient was taken to the EP Laboratory. Access was via an 8-Fr sheath in the right femoral vein with a 10-Fr oesophageal catheter used as the reference for the EnSite Precision™ Cardiac Mapping System. Three-dimensional reconstruction of the right atrium revealed a single atrial focus identified at the posterolateral right atrium with an atrial electrogram 34 ms ahead of the surface P wave and a unipolar signal with a pure Q wave. Attempts to place radiofrequency energy with a St. Jude Daig SAFIRE 4-mm radiofrequency (RF) catheter were unsuccessful secondary to a power <5 W. As such, the RF catheter was withdrawn, and a Medtronic CryoFreezor 6-mm XTRA1 catheter was advanced to the identical spot on the three-dimensional NAVX map. On the first cryoablation lesion, the tachycardia abruptly terminated to sinus rhythm as the temperature approached −50 °C. Two additional 4-minute insurance lesions were placed around the successful site. Atrial pacing with programmed electrical stimulation carried down to 400/200 ms resulted in no inducible atrial tachycardia. The atrial endocardial electrograms in the right atrium post-ablation were healthy appearing without any concerning widespread fractionation. The patient remained in sinus rhythm on VA ECMO for 7 hours at which point he went back into another ectopic atrial rhythm with a slightly different but singular P wave axis and morphology. No additional anti-arrhythmic medications were utilised aside from continuing the ivabradine and the amiodarone. There were no additional boluses of amiodarone or change in the infusion rate. Over the next 12 hours, the patient remained in a paroxysmal ectopic atrial tachycardia. A decision was made to go back to the EP Laboratory. Using the St. Jude EnSite Precision™ Cardiac Mapping System, another single atrial focus was identified in the superior right atrium posterior to the typical location of the sinus node. The sinus node had been mapped at the conclusion of the first ablation. The local atrial electrogram activation was 38 ms ahead of the surface P wave with a deeply negative pure Q wave on the unipolar signal. On the first cryoablation lesion, at −60 °C, the tachycardia terminated and the patient was in sinus rhythm. One additional insurance cryoablation lesion was placed. Attempts to place additional insurance lesions were limited as the patient developed junctional rhythm during the cryothermy lesion. Using a 10-Fr oesophageal EP catheter with burst atrial pacing and programmed electrical stimulation with S2 and S3 stimuli did not invoke any atrial arrhythmias. Mapping of the left atrium including the right pulmonary veins was not performed as there was no patent foramen ovale (PFO) and the observation of a signal more than 35 ms ahead of the surface P wave, excellent unipolar signal, and termination of the tachycardia with a single cryo lesion supported what was deemed to be a single right atrial focus. The patient remained in sinus rhythm overnight and was decannulated from VA ECMO the following morning. An echocardiogram 24-hour post-decannulation from ECMO revealed an LV ejection fraction of 48% supporting the diagnosis that this was a tachycardia-induced cardiomyopathy and not a primary cardiomyopathy. Ivabradine was stopped 3 days after ECMO decannulation and following extubation. The patient remained in sinus rhythm on oral amiodarone and beta-blockers and off all intravenous heart failure medications. Nine months post-ablation on monotherapy with beta-blockers, the patient is clinically doing well with normal ventricular function and in sinus rhythm with occasional premature atrial beats and atrial couplets.
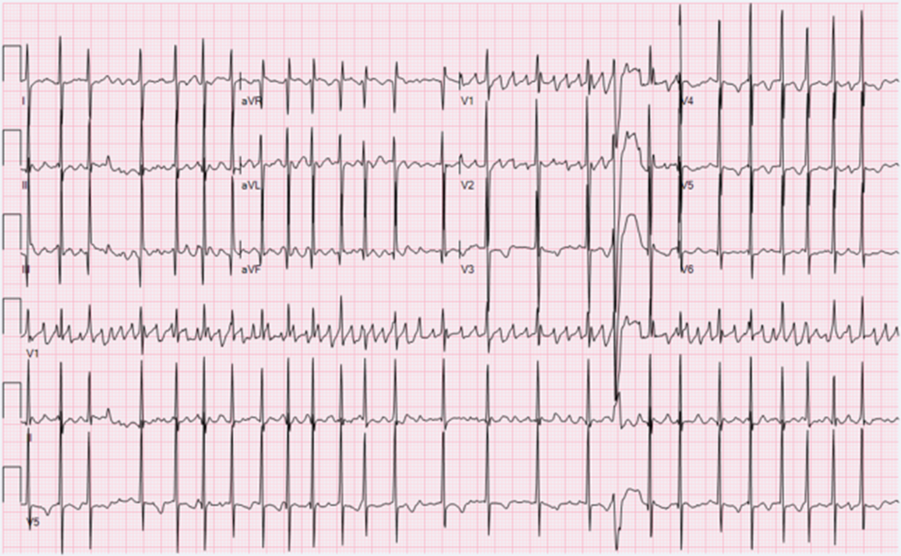
Figure 1. Twelve-lead electrocardiogram on arrival showing a rapid tachycardia with multiple different P waves. The P–R, R–R, and R–P all vary. The wide complex beats likely reflect some level of aberrancy. The P waves are most obviously disparate in leads I and II.
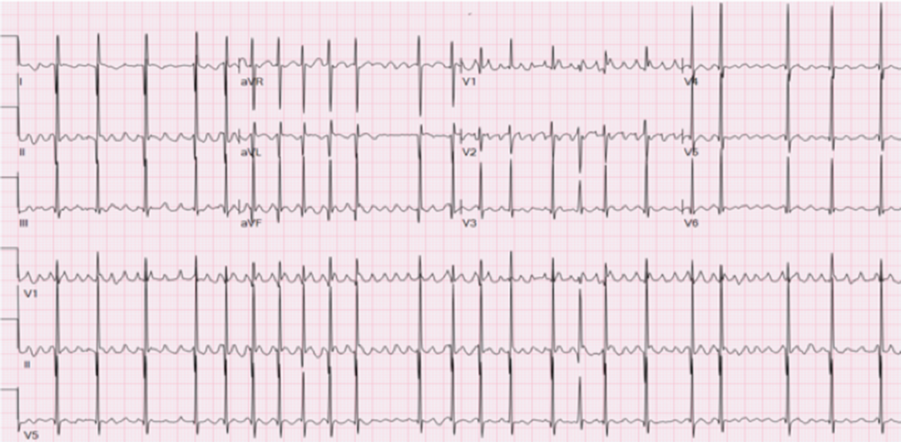
Figure 2. Twelve-lead electrocardiogram after two oral doses of ivabradine, prior to the first ablation, revealing a predominantly single ectopic atrial focus with variable ventricular conduction. Note the organisation of the P wave in lead II after ivabradine compared to the P wave during the initial tachycardia.
Discussion
Multifocal atrial tachycardia is defined as a tachycardia with at least three morphologically distinct P waves with irregular and varying P–P, P–R, and R–R intervals, rapid ventricular rates (150–250 bpm) with marked irregularity. At the higher atrial rates, aberrant ventricular conduction might appear to support ventricular tachycardia. Interspersed with these rare runs of tachycardia are pauses, blocked premature atrial beats. The vast majority of children with multifocal atrial tachycardia present under a year of age with 24–43% having some associated respiratory illness.Reference Bradley, Fischbach, Law, Serwer and Dick1,Reference Baek, Chung, Song, Bae, Kim and Ci2 In a series of 21 patients (median age 1.8 months) with multifocal atrial tachycardia, Bradley reported that 27% had diminished ventricular function with two being critically ill.Reference Bradley, Fischbach, Law, Serwer and Dick1 A variety of anti-arrhythmic medications were utilised with a mixed response though the natural history was fairly favourable with 52% in sinus rhythm within 5 months and 94% asymptomatic and free of any arrhythmia by 60 months.Reference Bradley, Fischbach, Law, Serwer and Dick1 This is in contradistinction to our patient who presented at a slightly older age, 5 months, in extremis and required advanced mechanical circulatory support. While the initial electrocardiogram could have been interpreted as atrial fibrillation, it would have been very unusual not to respond to multiple attempts at cardioversion.
Ivabradine is a newer anti-arrhythmic agent predominantly used in the heart failure arena to treat inappropriate sinus tachycardia. Ivabradine acts via selective inhibition of the funny current responsible for the spontaneous depolarisation of cardiac pacemaker cells. Ivabradine has been utilised for the treatment of both ectopic atrial tachycardia and junctional ectopic tachycardia in children.Reference Dieks, Klehs, Muller, Paul and Krause3–Reference Janson, Tan, Iyer, Vogel, Vetter and Shah5 Both of these dysrhythmias have a mechanism of action based on increased automaticity. The drug is especially appealing in patients who have haemodynamic instability as ivabradine has a rather haemodynamically neutral profile compared to other intravenous anti-arrhythmics. For this reason, Ivabradine has also been utilised in children with dilated cardiomyopathy and stable heart failure with a low adverse event rate.Reference Bonnet, Berger, Jokinen, Kantor and Daubeney6 However, this is the first report showing the beneficial effect of ivabradine in a child in extremis with ventricular dysfunction secondary to a rapid uncontrolled multifocal atrial tachycardia. The use of ivabradine in this particular case allowed for a multifocal atrial tachycardia to be converted to a single ectopic atrial pattern facilitating catheter ablation while on ECMO. While a second ablation was performed the following day again with a new but single ectopic focus, it appeared that the ivabradine had some effect on the automaticity and stabilisation of the multifocal atrial tachycardia.
The mechanism of multifocal atrial tachycardia has been postulated to be a dysrhythmia with many atrial ectopic foci, thus explaining the multiple P wave morphologies. Consideration that the arrhythmia is the result of a singular ectopic focus with varied levels of atrial propagation has been previously postulated.Reference Bevilacqua, Rhee, Epstein and Triedman7 In a prior case report, Bevilacqua described a 4-month old with multifocal atrial tachycardia and depressed ventricular function who responded to a single radiofrequency application at the posterior margin of the fossa ovalis in the right atrium.Reference Bevilacqua, Rhee, Epstein and Triedman7 The initial baseline electrocardiogram pattern on arrival to the hospital of our patient is similar to atrial fibrillation. Atrial fibrillation may arise from a single rapidly firing driver with fibrillatory propagation or by producing multiple fibrillatory re-entrant circuits.Reference Iwasaki, Nishida, Kato and Nattel8 The exact mechanism that explains the response of our patient and the adult with paroxysmal atrial fibrillation to ivabradine is unknown. It can be postulated that there is some cellular electrophysiologic slowing effect from ivabradine either locally at the ectopic focus itself or slowing downstream atrial fibrillatory conduction by changes in automaticity. In this small infant, one cannot exclude that the catheter ablation actually affected the right atrial ganglionated plexi, an observation that has been reported in adults with paroxysmal atrial fibrillation.Reference Mecca, Hongsheng, Telfer and Olshansky9,Reference Calo, Rebecchi and Sciarra10 Ongoing published clinical success with ivabradine for a number of supraventricular arrhythmias will hopefully extend the clinical indications of using this novel anti-arrhythmic agent.
Study limitations
Although no immediate effect was observed with the administration of amiodarone, it is possible that ivabradine’s effect on the tachycardia is related to a combination with amiodarone or a delayed effect from intravenous amiodarone. It is possible that using ivabradine for a longer duration in conjunction with another anti-arrhythmic agent such as a beta-blocker or amiodarone would have provided stable rate control in the setting of an ectopic atrial rhythm. Given the fact that the patient remained on ECMO with persistent ectopic atrial tachycardia a repeat ablation was performed. Volumetric changes in the right atrium on VA ECMO or cannula placement could have also resulted in some change in the arrhythmic focus though the effect was not seen upon initiation of VA ECMO. The distance between the first and second cryoablation lesions was approximately 5 mm in this 7-kg infant, and it is possible that the first lesion was slightly inferior to the ultimately successful site and that local injury transiently affected the focus. The proximity of the venous ECMO cannula to the RF ablation catheter likely resulted in the low power observed during the initial RF lesion.
Conclusion
This is the first case report that enteral ivabradine administered to a critically ill infant with refractory multifocal atrial tachycardia organised atrial conduction patterns confirming this likely originated from a single ectopic focus and thereby facilitated three-dimensional EP mapping and successful catheter ablation.
Key teaching points
Multifocal atrial tachycardia can present with severe ventricular dysfunction requiring advanced mechanical support.
Ivabradine has been utilised to treat both junctional ectopic tachycardia and ectopic atrial tachycardia but should be considered in the anti-arrhythmic armamentarium of multifocal atrial tachycardia.
Multifocal atrial tachycardia in infants under 6 months of age may reflect a primary ectopic atrial tachycardia with a fibrillatory exit pattern resulting in multiple P waves.
Acknowledgements
We would like to thank the wonderful group of nurses in the pediatric intensive care unit and the electrophysiology laboratory at Inova Children’s Hospital for their wonderful care of the patient discussed.
Financial Support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
There are no conflicts of interest for M.C., J.C., L.S., and L.C. C.S. is employed by Abbott Medical.




