After 16 years from the very first percutaneous valve implantation in the pulmonary position, this method of treatment for conduit valve dysfunction is, nowadays, considered established. The two most popular pulmonary valves in use have shown excellent short- and long-term results.Reference Lurz, Bonhoeffer and Taylor 1 – Reference Holzer and Hijazi 3 The majority of percutaneous valve implantations are performed through the transfemoral approach and only a small number of valves are implanted through the jugular vein.Reference Lurz, Bonhoeffer and Taylor 1 , Reference Zampi, Berman and Bocks 4 – Reference Berman, McElhinney, Vincent, Hellenbrand and Zahn 6 Variations in the cardiac and vascular anatomy may create technical problems during the process of insertion of the valves.Reference Zampi, Berman and Bocks 4 We report the first case of a successful percutaneous pulmonary valve implantation in a patient with a rare combination of vascular and cardiac anatomy.
Case description
A 14-year-old female with tetralogy of Fallot, mesocardia, hemiazygos continuation of the inferior caval vein, a dominant left-sided superior caval vein draining into the coronary sinus, and an absent right superior caval vein underwent, because of severe cyanosis, a modified Blalock–Taussig shunt procedure when she was a newborn. Later on, at the age of 4 months, she underwent a total correction with ventricular septal defect closure, a homograft insertion between the right ventricle and the pulmonary artery, and patch augmentation of the left pulmonary artery. Because of restrictive physiology of the right ventricle, a small atrial communication was created. Thereafter, the patient underwent numerous catheterisations because of a stenosis of the left pulmonary artery and, at the age of 5 years, she underwent device closure of the atrial communication.
When she was 12 years old, the first balloon dilatation of a homograft stenosis was performed and, 1 year later, she underwent a stent implantation – covered CP 8Z34 from NuMED Inc. over a 20-mm Z MED II Balloon from NuMED Inc. – because of recurrent conduit stenosis, which was attempted via a left-sided jugular vein approach. After relieving of the stenosis, she was scheduled for a percutaneous pulmonary valve implantation because of free homograft valve regurgitation. During the same session as the percutaneous pulmonary valve implantation, a second stent – AndraStent XL, 30 mm, from Andramed over a 15-mm Z MED II Balloon – into the left pulmonary artery and an additional stent – AndraStent XXL, 39 mm, over a Z MED II Balloon 22 mm – in the homograft, distal to the first one, were implanted in order to adequately prepare the landing zone for pulmonary valve implantation.
Finally, a 22 mm Melody® Valve was successfully implanted. All haemodynamic parameters after the valve implantation showed an optimal result. The procedure was performed under deep sedation, without general anaesthesia, according to the standard protocol in our unit.Reference Hanslik, Moysich, Laser, Mlczoch, Kececioglu and Haas 7
Cardiac anatomy and technical details
The patient’s vessel and intracardiac anatomy made the process of valve implantation challenging. A right superior caval vein was absent; instead, there was a left superior caval vein, which was draining into a wide coronary sinus at the left side of the heart. The right atrium was, however, on the right side in the chest and the right ventricle was anterior and in the middle line (Fig 1a, b). The catheters (Fig 2a, b) as well as the long sheath for the stent implantation (Fig 3a, b) and the Melody valve “ensemble” (Fig 4) were introduced through the left superior vena cava to the coronary sinus and to the right-sided right atrium. Subsequently, the whole assembly could be advanced through the tricuspid valve to the outflow tract of the anterior-sited right ventricle.

Figure 1 (a) Anteroposterior view: angiography in the left superior caval vein shows its connection to a dilated coronary sinus. Atrial septum is designated by the atrial septal occluder, which was implanted in a previous procedure. (b) Lateral view: angiography in the left superior caval vein shows it to be draining into a dilated coronary sinus. Atrial septum is designated by the atrial septal occluder, which was implanted in a previous procedure.
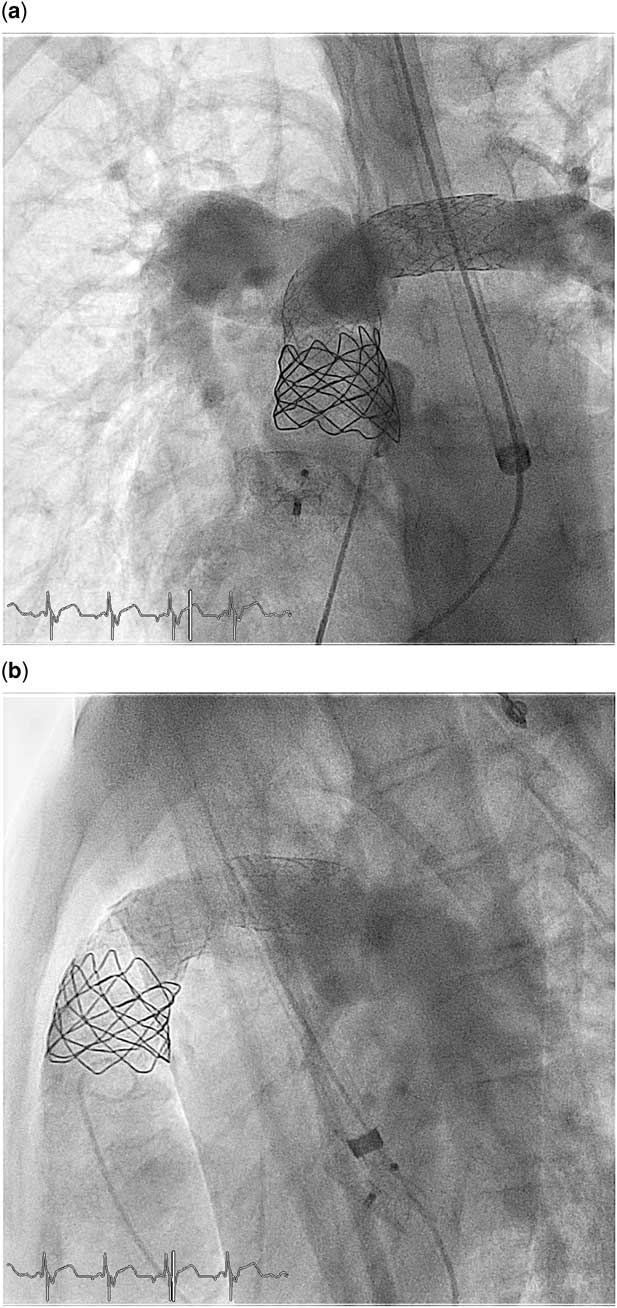
Figure 2 (a) Anteroposterior view: the descending and ascending parts of the catheter are in different coronal planes. Angiography after the stent implantation in the left and main pulmonary artery shows the perfect stent position after implantation as preparation for the Melody valve. (b) Lateral view: lateral shows the “U”-type configuration of the catheter although the access is through the left superior caval vein–coronary sinus and not through the right superior caval vein–right atrial loop. Angiography after stent implantation in the left and main pulmonary artery shows the perfect stent position after implantation as preparation for the Melody valve.

Figure 3 (a) Anteroposterior view: the positioning of the Melody valve in the landing zone. (b) Lateral view: the positioning of the valve in the landing zone.

Figure 4 Anteroposterior view: angiography in the main pulmonary artery after valve implantation. Absence of valve regurgitation.
On the basis of the specific anatomy of our patient with the absence of a right superior caval vein, accessing the transjugular vein through the left superior vena cava and coronary sinus to the right atria and then to the right ventricle with mesocardia resulted in a “U-spiral” type of pathway. Therefore, we created a pre-formed extra-stiff guide wire (Lunderquist® Extra-Stiff Wire Guide; Cook Medical Inc.), which was placed in the left pulmonary artery (Fig 4). The manually formed extra-stiff guide wire as well as the large delivery sheath of the Melody “ensemble” kept a smooth and undistorted shape, in order to deliver the valve to the indicated position and to make the final adjustments before the implantation. These manoeuvres made the approach to the right ventricle outflow tract smooth without provoking distortion to the anatomy and to the function of the tricuspid valve during the procedure. The “ensemble” remained in stable position during balloon inflation and the valve positioning was precise in the landing zone. The Melody® valve showed perfect functioning after the implantation without any regurgitation (Fig 4). Continuous monitoring of the arterial pressure revealed stable haemodynamics throughout the procedure.
The duration of the whole procedure was 172 min and the radiation time was 24.9 min – radiation dose 6.197, 3 cGy cm2, weight 46 kg, height 152 cm – and, thereby, within reported limits.Reference Zampi, Berman and Bocks 4 , Reference Eicken, Ewert and Hager 8
Discussion
The largest series of percutaneous pulmonary valve implantation in the literature report on valve implantation from a femoral vein access route.Reference Lurz, Bonhoeffer and Taylor 1 , Reference Zampi, Berman and Bocks 4 – Reference Berman, McElhinney, Vincent, Hellenbrand and Zahn 6 Few reports in the literature support access through the right jugular vein as being feasible and as safe as the femoral access route for percutaneous pulmonary valve implantation; in some individual cases the access route was changed during the procedure from the femoral vein to the jugular vein.Reference Zampi, Berman and Bocks 4 – Reference Berman, McElhinney, Vincent, Hellenbrand and Zahn 6 There are no reports, to the best of our knowledge, of literature that describe an approach to percutaneous pulmonary valve implantation through a left superior caval vein–coronary sinus pathway in a patient with this kind of anatomy. Of course, a transhepatic approach could have overcome the special anatomic features of our patient, but it would increase the risk for complications. Having already approached the patient once from the left superior caval vein route for stenting the origin of the left pulmonary gave us the confidence of following the same pathway for percutaneous pulmonary valve implantation.
On the basis of the lateral view projections (Figs 2b and 3b), the wire and the Melody “ensemble” kept the described “U-spiral”-type configuration. The “U-spiral” shape is of the same configuration as for the access from the right superior jugular vein. On the basis of the frontal view projection (Figs 3a and 4) the wire and, consequently, the long sheath and the valve “ensemble” show a “spiral” configuration with the descending part and the ascending part of the wire being in different planes. To achieve this, the extra-stiff exchange wire had to be manually shaped in these two directions according to the underlying anatomy to enable a smooth and safe placement of the long sheaths and delivery systems. This configuration provided us the proper support and accessibility in order to implant a stent in the origin left pulmonary artery, to dilate the already existing stent and implant a new one, in order to create a long landing zone for our valve implantation (Figs 2a, b, 3a, b, 4). The duration of the procedure and the radiation time was comparable to those reported in the literature,Reference Zampi, Berman and Bocks 4 , Reference Eicken, Ewert and Hager 8 facts that support the feasibility of the process and of the approach.
The jugular route of access for percutaneous pulmonary valve implantation seems to solve access difficulties, which could have emerged during the procedure not only from a relatively small right ventricle or the angle of the outflow tract in patients weighing <30 kgReference Holzer and Hijazi 3 – Reference Berman, McElhinney, Vincent, Hellenbrand and Zahn 6 but also for patients with obscure and challenging anatomy, which exclude the femoral vein access.
Of course, as we report on only one patient, because of the rare existence of such an anatomy, is difficult to conclude the selection criteria and guidelines for future cases. Our intent was to show that the presence of an uncommon anatomy alone should not be an exclusion criterion for percutaneous pulmonary valve implantation.
Conclusion
On the basis of this unique experience, we believe that percutaneous pulmonary valve implantation may be possible even in patients with unusual and challenging anatomy. Establishing manually shaped specific forms of the extra-stiff exchange wires according to the underlying anatomy is a helpful tool and crucial to enable a safe valve positioning, especially in these patients.
Acknowledgements
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Financial Support
No grants were used other than funding from the hospital.
Conflicts of Interest
None.







