The ductus arteriosus (DA) is derived from the distal dorsal sixth aortic arch, in order to shunt blood to bypass the pulmonary circulation. The DA is patent by 8 weeks of gestation. The patent ductus arteriosus (PDA) allows the mixing of oxygen-rich blood from the descending aorta and the oxygen-depleted blood of the pulmonary artery. This is because the lungs are not used during gestation, as the fetus is directly supplied with oxygen from the placenta. The DA normally closes within 24–72 hours after a term birth due to the decrease in prostaglandin, prostacyclin, and oxygen tension, resulting in intraluminal ischaemic hypoxia, after which the DA becomes known as the ligamentum arteriosum. Whereby failure of closure within 72 hours results in the diagnosis of PDA. Reference Hermes-DeSantis and Clyman1 PDA is the most common congenital heart defect in preterm infants with the incidence ranging from 20 to 60%, whereas it comprises of 5–10% in term infants. Reference Schneider and Moore2 PDA in preterm infants is linked with immaturity, whereas PDA in term infants is associated with functional defects. Reference Dice and Bhatia3 PDA is also highly correlated with reduced mortality and morbidity; therefore, a common solution has been the closure of PDA. Multiple treatment modalities are available from medical to surgery, though contributing to uncertainty regarding choice. Other uncertainty surrounds the effectiveness of PDA closure and whether a medical or surgical closure would have a significant effect on morbidity and mortality. This review aims to highlight the current literature on clinical presentation, management methods, intervention strategies, and risk assessment of PDA to aid clinicians. Furthermore, pathophysiology and future research opportunities are also introduced and briefly summarised.
Pathophysiology
The ductus arteriosus (DA) is important in foetal circulation as it stops the blood from reaching the non-functional foetal lungs by connecting the main pulmonary artery and the descending aorta, allowing blood with a low oxygen concentration to flow through the descending aorta to the placenta, for gas exchange. Ninety percentage of right ventricular output flows through the DA before birth. Reference Dice and Bhatia3 The patency of the foetal DA is maintained by the low foetal oxygen tension and the action of COX enzymes in forming circulating prostaglandins that vasodilate the ductus arteriosus. DA smooth muscle relaxation is the result of the activation of G-coupled prostaglandin receptor EP4 by PGE2, the most common product of arachidonic acid metabolism. The activation of EP4 leads to the build-up of cyclic adenosine monophosphate, a low myosin light-chain kinase and an increase in protein kinase A, which cause vasodilation and ultimately DA patency. In the final period of gestation, smooth muscle cells within the DA migrate and form a round mass known as the intimal cushions towards the endothelial lining. At birth, a sudden decrease in prostaglandin levels caused the constriction of the DA, by the merging of the intimal cushions to obstruct the DA lumen. Reference Smith4 The ductus in a preterm foetus is even more sensitive to the effects of prostaglandins in vasodilation, therefore increasing the risk of ductal closure failure. On the other hand, term infants have a decreased sensitivity of the DA to prostaglandins and decreased circulating levels of PGE2 contribute to DA closure. Reference Clyman, Mauray, Rudolph and Heymann5 Patent ductus arteriosus is when the ductus arteriosus fails to close after birth, consequently, leading to the formation of a left-to-right shunt, since the blood pressure in the aorta is much higher than the pulmonary artery. Patency increases the pressure inside the pulmonary circuit leading to pulmonary hypertension, which causes arrhythmias and congestive heart failure. Patent ductus arteriosus could sometimes be induced to produce a positive outcome in children born with life-threatening congenital heart defects, for example, in children born with a transposition of the great vessels, where PDA can help maintain circulation. This is useful as it allows surgeons time to prepare for surgery. Reference Huff, Chaudhry, Arora and Mahajan6
Presentation of patent ductus arteriosus
The clinical presentation of PDA is dependent upon the size of the DA and gestational age.
Infants with a small PDA can remain asymptomatic or may take some time for symptoms to progressively develop. The symptoms of PDA are mainly due to the left-to-right (aortic- pulmonary) shunt, resulting in excessive perfusion of the pulmonary vasculature. The progressive congestion ultimately causes respiratory failure and the lack of systemic perfusion results in end-organ damage. End-organ damage manifests as renal failure, in addition, infants may also develop feeding intolerance, necrotizing enterocolitis, intraventricular haemorrhage, periventricular leukomalacia, and cerebral palsy. Reference Drougia, Giapros and Krallis7 Infants present with episodes of apnoea, signs of heart failure such as tachypnoea, dyspnoea with feeding, tachycardia, bounding pulse, and wide pulse pressure. Reference Mitra, de Boode, Weisz and Shah8 The infant progressively develops chronic lung disease and congestive heart failure requiring ventilator. Reference Gillam-Krakauer and Mahajan9
Risks of persistent patent ductus arteriosus
A persistent PDA in neonates and infants has historically been correlated with increased morbidity and mortality risks at 30%, with an increased incidence in preterm infants in comparison to their term counterparts, ranging from 20 to 60%. Reference Dice and Bhatia3
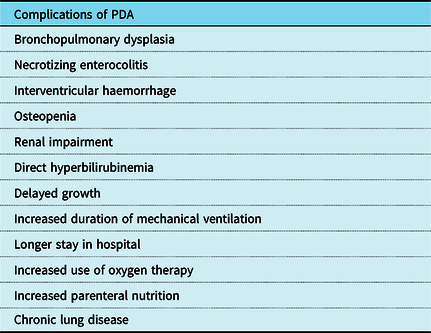
The physiological sequelae of a persistent PDA are well established, such as bronchopulmonary dysplasia (BPD), chronic lung disease (CLD), necrotizing enterocolitis (NEC), retinopathy of prematurity (ROP), heart failure, infective endocarditis, intraventricular haemorrhage, osteopenia, renal impairment, direct hyperbilirubinemia, and delayed growth (Table 2). Furthermore, there is an increase in the duration of mechanical ventilation, stay in hospital and use of oxygen therapy and parenteral nutrition, and tracheostomy whilst in hospital. Reference Saldeño, Favareto and Mirpuri10
A review reported that at follow-up, there was an increased association of CLD, neurodevelopmental impairment (NDI), and ROP amongst infants who underwent surgical ligation as opposed to the persistent PDA that were treated more conservatively. Reference McNamara and Weisz11
The extent of haemodynamic stability, gestational age, and birth weight seem to be pertinent factors in regard to clinical assessment of whether surgical interventions such as ligation or more conservative, “watchful waiting” and pharmacological management is required
Evidence from more recent studies indicates that there is eventual spontaneous closure in 80% of non-ligated infants, although the median age of closure is 42 days in comparison to 28 days in ligated infants. This suggests that where haemodynamically appropriate, persistent patency may be of a long-term benefit if surgical intervention and its subsequent complications can be avoided. Reference Sankar, Bhombal and Benitz12 However, in the correct clinical context, such as in the instance of systemic and pulmonary duct-dependent cardiac defects, maintaining patency of the DA is of utmost importance and may be lifesaving. Reference Deshpande, Baczynski, McNamara and Jain13
Interventions for persistent patent ductus arteriosus
Management options for a patent PDA can be medical or surgical. Indications for management include the onset of symptoms including respiratory compromise, concomitant heart failure, or a large left-to-right ductus shunt. As summarised in Table 3, various medical and surgical techniques have different risks and long-term outcomes.
Medical
Medical therapy can be used to help close a patent ductus arteriosus. These include NSAIDs such as indomethacin and ibuprofen along with paracetamol. When used on days 1–2 of postnatal life, there is a lower risk of pulmonary haemorrhage and intraventricular haemorrhage. Reference Gillam-Krakauer and Mahajan9 Such NSAIDs have been found to have a direct effect on increasing amiloride-sensitive alveolar epithelial sodium channels causing increased lung water clearance and as such reducing the risk of pulmonary oedema. Reference Clyman14
In duct-dependent cardiac lesions, medical therapy can be used to keep the ductus arteriosus open. This involves the use of prostaglandin E1 such as alprostadil and prostaglandin E2 such as dinoprostone. These both cause vasodilation and are given as a continuous IV infusion.
Modest fluid restriction and treatment of respiratory complications including pulmonary oedema through positive end-expiratory pressure can help supplement medical therapy.
Surgical
If there is a poor response or contraindication to medical treatment of DA closure, surgical options can be sought. The ductus arteriosus can be kept open through percutaneous stenting and surgical shunts traditionally (also known as the modified Blalock–Taussig (BT) shunt). These surgical approaches are widely used in palliative cases. Stenting was first performed in 1992 and has since been more popular than BT shunt.
Percutaneous stenting
In duct-dependent pulmonary circulation, percutaneous stenting can be employed in palliative cases to keep the DA open. It works by maintaining adequate SO2 and promotes balanced pulmonary artery growth. PDA stenting was first performed in 1992 and has been growing in popularity since. This is largely due to it being less invasive by avoiding a median sternotomy and allowing for shorter recovery times and reduced requirement for post-operative ECMO.
However, the transcatheter approach then introduces complications of vascular access and stent lifespan. If the stent diameter is too small, there is a risk of restenosis and ductal spasm. The stent can also become dislodged and migrate causing chamber damage or perforation. Acute stent occlusion due to stent thrombosis can also occur due to neointimal proliferation and fibrosis in ductal tissues extending into the media of the pulmonary artery wall. Ultimately, such cases require an aorto-pulmonary shunt.
Percutaneous closure
A transcatheter approach to closure of the DA is the preferred method in patients with non-duct-dependent pulmonary circulation. Frequently used devices include Gianturco coils, Amplatzer Septal Occluders, and Nit-Occlud device. Moderate-to-large PDAs can now be treated percutaneously too. Reference Sullivan, Theleman and Choi15 Complications can include device embolisation into the pulmonary artery and distal aorta. In adults, the onset of atherosclerotic changes in the aorta and access site can accelerate complication rates.
Surgical closure
Open heart surgery is usually performed for the correction of underlying defects including arterial switch operation, truncus arteriosus repair, and VSD closure. Surgical ligation and division was the gold standard for PDA treatment until percutaneous closure was developed. There are two principal methods: closure by clip and via ligation. The clip method involves a left thoracotomy in the third–fourth intercostal space. After the duct is dissected, a clip of appropriate size is placed around the duct and closed. During the ligation method, the ductus is mobilised and the ligature is passed around it to tie it down. Whilst surgical closure is associated with longer recovery time, there is a very low mortality and restenosis risk. Reference Valentík, Omeje, Poruban, Sagát and Nosál16 Surgical closure techniques can also be a conventional transpleural approach or via a posterior minithoracotomy extrapleural approach. However, there are no statistically significant differences between the two methods in acute 30-day mortality as evidenced by Alvarez et al. Reference Avila-Alvarez, Serantes Lourido, Barriga Bujan, Blanco Rodriguez, Portela-Torron and Bautista-Hernandez17
In palliative patients, a surgical alternative to percutaneous stenting is the modified Blalock–Taussig (BT) shunt. This is a surgical procedure used to increase pulmonary blood flow in duct-dependent cyanotic heart defects. Through a sternotomy approach, a graft is inserted between the subclavian and pulmonary arteries. Reference Odim, Portzky and Zurakowski18 Complications of the modified BT shunt are instead related to invasive surgery with thoracotomy and median sternotomy. These can include pleural effusions, phrenic nerve palsy, and increased post-operative length of stay.
When to keep PDA open
Usually if the DA fails to close after birth, medical management can help facilitate closure. However, certain duct-dependent flow defects rely on a patent DA for survival. These defects are termed critical congenital heart defects. The presence of central shunts (ductus arteriosus, ductus venosus, and foramen ovale) is important for effective fetal and postnatal circulation. Most patients with such defects have severely hypoplastic right or left ventricles and/or severe obstructive valvular or outflow tract defects. Presentation with dyspnoea and cyanosis will typically occur within hours or days after birth. Examples of right-to-left shunt lesions can include tetralogy of Fallot, pulmonary atresia with ventricular septal defect, Ebstein’s anomaly, or defect with severe pulmonary stenosis or pulmonary atresia. Similarly, left-sided obstructive lesions requiring a patent ductus arteriosus for survival include hypoplastic left heart syndrome, severe aortic valve stenosis, coarctation of aorta, or defect with systemic outflow obstruction.
Whilst initially, medical management with prostaglandin E1 infusions can help keep the DA open, stents can be inserted for longer duration effect. These are largely used in the palliative care setting.
Keeping the DA open in such congenital heart defects is the only means of blood supply to the body. For instance, in pulmonary atresia, there is reduced or absent blood flow from the right ventricle to the pulmonary circulation and so a patent foramen ovale and ductus arteriosus allows for shunting of blood across the atria and pulmonary circulation respectively for survival.
Discussion
Both PDA stenting and modified BT shunt are popular options to help treat duct-dependent cardiac lesions. However, various studies have been performed comparing the efficacy between these options. Multiple multicentre studies provide corroboration for superior efficacy and reduced morbidity associated with PDA stenting (Table 3 and 4). Alwi et al, 2000 studied 69 patients with a duct-dependent pulmonary circulation undergoing PDA stenting during a 3-year period and found a 91% success rate. Reference Alwi, Choo, Latiff, Kandavello, Samion and Mulyadi19 Glatz et al Reference Glatz, Petit, Goldstein and Qureshi20 compared outcomes in 106 patients with a PDA stent and 251 patients with a BT shunt in a multicentre study. With differences in patient factors adjusted for, there was no difference in the primary end point of death, however, there was reduced morbidity and pulmonary artery size in the PDA stenting group favouring this approach in selective patients. The reduction in morbidity was represented as reduced length of stay in the hospital, post-operative complications, and reduced requirement for medical therapy such as diuretics (Table 1).
Table 1. Summary based on outcomes from Glatz et al (2017)

Table 2. Complications of persistent PDA

Table 3. Summary of literature comparing PDA stent and BT shunts in duct-dependent pulmonary circulation

Table 4. Summary table comparing percutaneous closure with surgical closure in non-duct-dependent cardiac lesions

The popularity of PDA stenting as an alternative to BT shunting is also a result of improvements in stent design and clinician experience. These have helped in the correct positioning of the PDA stent for enhanced clinical efficacy (Table 3). For example, Gewillig et al, 2004 found increasing the diameter of the premounted coronary stent from 3.0 to 4.0 mm helped cover the duct completely and provided symptomatic relief for a further 3–4 months. Reference Gewillig, Boshoff, Dens, Mertens and Benson21
Whilst PDA stents offer reduced morbidity and equivocal mortality benefits, there are some limitations to be aware of. The durability of stents can be impacted by complications such as thrombosis and neointimal proliferation. This can be overcome through the use of drug-eluting and heparin-coated stents. In Alwi et al’s study, thrombosis complicated one patient’s recovery at 3 weeks post-stenting and in six patients within 6 months. Reference Alwi, Choo, Latiff, Kandavello, Samion and Mulyadi19 This was evidenced by a reduction in oxygen saturation. Aggarwal et al 2019 compared outcomes of drug-eluting stents (DES) versus bare-metal stents (BMS) specifically in cases of infant PDA stenting in a retrospective study between 2004 and 2018. DES treatment arm had reduced unplanned reinterventions (12 versus 28%, p = 0.03) for cyanosis and less luminal loss compared to BMS arm. Reference Aggarwal, Dhillon, Penny, Gowda and Qureshi22
In both PDA stenting and BT shunt procedures, mortality can be affected by patient variables. One variable is infant birth weight. Garg et al 2018 found three times higher mortality risk with BT shunt procedure in low body weight infants compared to normal body weight infants. In infants with a birth weight of <2 kg, PDA stenting may offer a safer alternative. Low birth weight is associated with prematurity, which also is a patient variable affecting mortality rates. Weight is such a strong prognostic indicator despite improvements to percutaneous technique and shunt materials. Reference Garg and Mittal23 Similarly, Petrucci et al 2011 found an inverse relationship between risk and weight. Reference Petrucci, O’Brien, Jacobs, Jacobs, Manning and Eghtesady24 Patients weighing <2.5 kg had a 15.6% mortality rate representing a 5× increase compared to those heavier than 3.5 kg. Interestingly, variables including age, gender, and ethnicity have not shown any mortality association with PDA stent and modified BT shunt procedures.
Prior to surgery, cardiac duct morphology needs to be established to confer eligibility for stenting or shunting. For example, the presence of a long tortuous duct, insufficiently constricted ductus at the pulmonary end and associated pulmonary stenosis at the site of stent insertion preclude a poor response to stenting.
An end point to determine efficacy that can be compared between stent versus non-stent treatment options is pulmonary artery growth. Santoro et al in 2009 studied this and found similar rates of growth (as measured by objective growth charts such as Nakata index) between PDA stent and BT shunt treatment arms. Similar to the end point of mortality, PDA stent arm has more symmetric growth of pulmonary arteries as evidenced by the left pulmonary artery and right pulmonary artery diameter ratio. Reference Santoro, Capozzi and Caianiello25
Whilst PDA stenting offers an attractive first-line option, the presence of pulmonary stenosis at the site of ductal insertion in select patients is an absolute contraindication as stenting can accelerate this. This may be explained by the stent provoking neointimal proliferation and fibrosis in ductal tissues encircling the pulmonary artery wall. Such patients are likely to be those with single ventricles and for whom Fontan procedures are necessary. This also epitomises the importance of cardiac catheterisation prior to stenting to elucidate the vascular anatomy. This is also a risk in a BT shunt given that blood flow typically enters the pulmonary circulation asymmetrically, thus, exacerbating stenosis at the site of shunt insertion.
Whilst stenting and shunting offer improved quality of life in palliative patients, it is also important to note the long-term effects of substantial left-to-right shunting. These include an increased risk of intraventricular haemorrhage, necrotizing enterocolitis, and bronchopulmonary dysplasia.
Future research
Although there are several interventions available for the management of PDA, the majority of the studies compared only two interventions. Therefore, a future RCT that includes all interventions would aid in decision-making for clinicians. Future studies are also needed to ascertain which infants are at greater risk of persistent PDA and which specific treatment they require. Essentially defining the higher risk category in terms of clinical and genetic profile and subsequent indications.
Studies should also use similar definitions for symptomatic PDA, interventions, indications for interactions, and what constitutes successful treatment of PDA looking at long-term outcomes. Finally, in terms of quality, the evidence should be evaluated using tools such as Grading of Recommendations, Assessment, Development and Evaluations (GRADE) for more objective comparisons.
Conclusion
A patent ductus arteriosus is necessary for foetal and neonatal life when there are significant duct-dependent congenital cardiac diseases. Yet, its persistence beyond a certain time can be harmful and therefore, it should be addressed. Both percutaneous and surgical approach offers satisfactory results and larger study data are required to set optimised guidelines.
Acknowledgements
None.
Financial Support
None.
Conflict of interest
The authors declare no conflict of interests.