Hypoxia is a universal feature of single ventricle congenital heart disease (CHD). The specific causes of hypoxia vary over time for patients as they undergo multiple staged surgical palliations. After palliation with a superior cavopulmonary connection, there is hypoxia due to obligate right-to-left shunting of venous blood from below the diaphragm. After subsequent Fontan palliation, no residual right-to-left shunting is present except for coronary sinus blood and a potential Fontan fenestration. However, intrapulmonary shunting through pulmonary arteriovenous malformations may develop, worsen, or regress after Fontan palliation and can variably impact oxygen saturation.
The prevalence of pulmonary arteriovenous malformations in single ventricle CHD is variably reported – ranging from 15 to 100% depending on the specific cohort, diagnostic criteria, and follow-up duration. Reference Bernstein, Brook, Silverman and Bristow1–Reference Vettukattil, Slavik and Lamb3 Many studies focus on the prevalence of pulmonary arteriovenous malformations in patients with heterotaxy syndrome and interrupted inferior caval vein because a superior cavopulmonary connection with interrupted inferior caval vein (i.e., Kawashima) creates near-Fontan physiology with only hepatic venous blood excluded from the pulmonary circulation. Reference Srivastava, Preminger and Lock2,Reference Shah, Rychik, Fogel, Murphy and Jacobs4
To assess resolution of pulmonary arteriovenous malformations after hepatic vein incorporation, previous studies monitored oxygen saturation changes after Fontan palliation. Reference Shah, Rychik, Fogel, Murphy and Jacobs4–Reference Alsoufi, Rosenblum and Travers9 These studies naturally concluded that increases in oxygen saturation months after Fontan palliation were due to pulmonary microvascular remodeling with resolution of pulmonary arteriovenous malformations, whereas continued low saturations were due to failure of resolution of arteriovenous malformations. However, these studies focused almost exclusively on patients with heterotaxy syndrome and interrupted inferior caval vein. These studies did not identify the burden of pulmonary arteriovenous malformations or oxygen saturation changes in other cohorts of patients with single ventricle CHD. It is unknown if pulmonary arteriovenous malformations develop equally before Fontan palliation and if oxygen saturations universally increase after Fontan discharge. Thus, the primary objective of this study was to determine whether pulmonary arteriovenous malformations are diagnosed more frequently before Fontan palliation in patients with heterotaxy syndrome compared to matched non-heterotaxy patients. Secondarily, based on previous studies, we sought to compare oxygen saturation changes after Fontan discharge as a clinical marker of resolution of pulmonary arteriovenous malformations.
Materials and methods
Patient selection
We conducted a retrospective chart review of patients seen at Children’s Wisconsin from 1999 to 2019. This time frame was chosen because of digital access to medical records. Inclusion criteria included a history of single ventricle CHD and Fontan palliation. From the list of patients satisfying our inclusion criteria, we identified patients with heterotaxy syndrome if 1) it was reported as a diagnosis in the medical record, 2) there was a known history of interrupted inferior caval vein, and/or 3) there was a known history of bilateral superior caval vein and heterotaxy syndrome was reported as “probable” or “likely” in the medical record. After identifying the heterotaxy cohort, we verified the anatomic status of the inferior caval vein by reviewing echocardiogram and cardiac catheterisation reports to sub-classify the heterotaxy group into either intact inferior caval vein (normal direct connection to the right atrium) or interrupted inferior caval vein. The heterotaxy group was then matched 1:1 by Fontan palliation date with a non-heterotaxy control group with a primary diagnosis of hypoplastic left heart syndrome. This study was approved by the local Institutional Review Board.
Pre- and post-operative analyses
Data regarding patient diagnoses, clinical variables, and pre- and post-Fontan oxygen saturation were obtained from the medical record. Diagnosis of pulmonary arteriovenous malformations pre-Fontan was obtained by reviewing the electronic medical record for a physician-documented diagnosis – either directly in the report of a contrast echocardiogram using agitated saline (“bubble study”), cardiac catheterisation report, or any diagnosis documented in a clinical note and signed by a paediatric cardiologist. Per our institutional practice, all bubble studies performed in patients with single ventricle CHD were performed during a cardiac catheterisation with direct injection of contrast into each branch pulmonary artery. Of those patients who had a bubble study performed pre-Fontan, images were independently reviewed by an attending cardiologist (ADS) to assess the severity of intrapulmonary shunting. Severity of shunting was classified as negative (no bubbles entering the single ventricle), mild (occasional or few bubbles filling of the single ventricle), moderate (moderate filling of the single ventricle), or severe (complete opacification of the single ventricle), per previously reported criteria. Reference Feinstein, Moore, Rosenthal, Puchalski and Brook10
Pre-Fontan oxygen saturation was obtained from the most recent outpatient saturation prior to Fontan palliation. Post-Fontan saturations were recorded at Fontan discharge and subsequent outpatient follow-up (3, 6, and 12 months Post-Fontan).
Statistical analysis
Cohort data are expressed as median (interquartile range [IQR]) for continuous data and N (%) for categorical data unless otherwise stated. We performed Pearson’s Chi-Squared or Fisher’s Exact test to compare categorical variables. We performed Kruskal–Wallis or Wilcoxon–Mann–Whitney tests to compare continuous variables between groups. A linear mixed model with normal distribution and identify link was performed to analyse oxygen saturations between and within groups. Diagnostic group, time, and interaction between group and time were included in the primary model. Additional models (sub-analyses) included pulmonary arteriovenous malformation diagnosis or bubble study severity in lieu of the diagnostic group. Models used maximum likelihood method, random effect of an intercept that could vary across subjects, and unstructured covariance structure for random effects. Residuals were examined to check model assumptions. Analyses were performed using SAS 9.4, SPSS 24.0, and GraphPad Prism 8 (GraphPad Software, San Diego, CA). Statistical significance was accepted at the level of p < 0.05. Tukey–Kramer adjustment was used for multiple comparisons.
Results
Patient characteristics
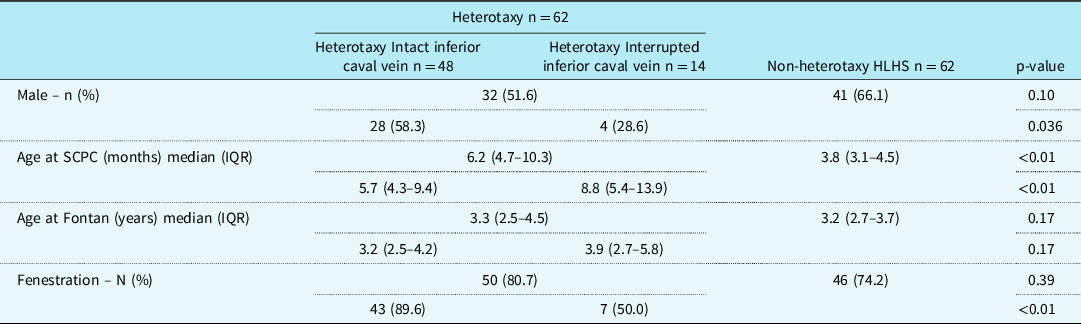
A total of 124 patients with single ventricle CHD and previous Fontan palliation were included in this study with 62 heterotaxy patients and 62 non-heterotaxy hypoplastic left heart syndrome controls. Of the 62 heterotaxy patients, 14 (22.6%) had an interrupted inferior caval vein. Patient demographics, including gender, age at superior cavopulmonary connection (Glenn or Kawashima), age at Fontan, and presence of a Fontan fenestration are summarized in Table 1.
Table 1. Patient demographics

HLHS = hypoplastic left heart syndrome; SCPC = superior cavopulmonary connection.
As expected, patients with heterotaxy syndrome and interrupted inferior caval vein had their superior cavopulmonary connection (Kawashima) performed at an older age compared to those patients with heterotaxy with intact inferior cava and compared to the non-heterotaxy control group (p < 0.01). Surprisingly, though, there was no statistical difference in age at Fontan palliation when comparing the main groups (heterotaxy versus non-heterotaxy, p = 0.17) and when comparing the heterotaxy sub-groups and non-heterotaxy control group (p = 0.17).
As described in Table 1, 96 patients (77.4%) underwent a fenestrated Fontan. Of those with a fenestration, five patients had closure (four spontaneous, one intentional) prior to discharge home after Fontan and seven more patients had spontaneous closure during the initial 12 months after Fontan palliation.
Pulmonary arteriovenous malformation diagnosis
Patients with heterotaxy and interrupted inferior caval vein were more likely to have a documented diagnosis of pulmonary arteriovenous malformations in their medical record prior to Fontan palliation (12/14; 85.7%) compared to patients with heterotaxy and intact inferior caval vein (10/48; 20.8%) and non-heterotaxy control (15/62; 24.2%) (p < 0.01) (Table 2). Similarly, patients with heterotaxy and interrupted inferior caval vein were more likely to have an echocardiogram with agitated saline (“bubble study”) performed pre-Fontan (10/14; 71.4%) compared to the other groups (heterotaxy intact inferior caval vein 6/48 (12.5%), non-heterotaxy controls 9/62 (14.5%); p < 0.01). Most, but not all, pulmonary arteriovenous malformation diagnoses were based on bubble studies (25/37; 67.6%). Non-bubble study diagnoses were documented on a pre-Fontan cardiac catheterisation (8/37; 21.6%) or pre-Fontan clinic note (4/37; 10.8%). All patients with a pre-Fontan bubble study had a positive result with at least mildly positive intrapulmonary shunting in one lung (15/25 (60.0%) severely positive, 10/25 (40.0%) mild or moderately positive). When reviewing all bubble studies for severity of intrapulmonary shunting, there was no difference in the frequency of a severely positive bubble study when comparing the primary groups (p > 0.99) and sub-groups (p = 0.17).
Table 2. PAVM diagnosis

HLHS = hypoplastic left heart syndrome; PAVMs = pulmonary arteriovenous malformations.
* Included only patients who had a bubble study performed pre-Fontan.
Oxygen saturation
The oxygen saturation had a skewness of −0.48 and kurtosis of −0.22. Using a linear mixed model, there was no difference in Pre-Fontan oxygen saturations among the three groups (heterotaxy: intact inferior caval vein 82.0 (78.0, 85.0)%, interrupted inferior caval vein 80.5 (77.0, 85.0)%; non-heterotaxy control 82.0 (79.0, 85.0)%; Fig 1). At Fontan discharge, the non-heterotaxy control group (90.0 (86.0, 94.0)%) increased their oxygen saturations more than both heterotaxy sub-groups (intact inferior caval vein 87.0 (83.0, 92.0)%, p < 0.01; interrupted inferior caval vein 84.0 (82.0, 86.0)%, p < 0.01), but there was no difference in oxygen saturation between the two heterotaxy sub-groups (p = 0.18). At 3, 6, and 12 months post-Fontan, there was no difference in oxygen saturation among the three groups at each time point.

Figure 1. Tukey Box and whisker plot comparing oxygen saturation changes before and after Fontan palliation based on the primary diagnostic groups – heterotaxy syndrome (intact inferior caval vein or interrupted inferior caval vein) and non-heterotaxy HLHS control group. Boxes represent the median and interquartile ranges (IQR); whiskers represent the 95% confidence interval; individual values represent values outside the 95% confidence interval. HLHS = hypoplastic left heart syndrome, ns = not statistically significant, SpO2 = peripheral oxygen saturation.
Because the oxygen saturations appeared to increase for all groups after Fontan discharge, we then analysed within-group changes in oxygen saturations both from the surgery itself (pre-Fontan versus Fontan Discharge) and after Fontan discharge (Discharge versus 3, 6, and 12 months Post-Fontan) (Fig 2). Patients with heterotaxy and intact inferior caval vein increased their saturations from Fontan palliation (p < 0.01) and at each follow-up time-point after Fontan discharge (3 months p = 0.04, 6 months p < 0.01, 12 months p < 0.01) (Fig 2a). Patients with heterotaxy and interrupted inferior caval vein did not increase their oxygen saturations from Fontan palliation (p = 0.09), but they did increase their saturations at each follow-up time-point after Fontan discharge (3 months p < 0.01, 6 months p < 0.01, 12 months p < 0.01) (Fig 2b). Finally, the non-heterotaxy hypoplastic left heart syndrome control group increased their saturations from Fontan palliation (p < 0.01) and with longer follow-up after Fontan discharge (6 months p = 0.02, 12 months p = 0.03) but not initial follow-up after Fontan discharge (3 months p = 0.24) (Fig 2c).

Figure 2. Tukey Box and whisker plots showing within-group comparisons of oxygen saturation changes before and after Fontan palliation for each diagnostic group. Patients with (a) heterotaxy syndrome and intact inferior caval vein, (b) heterotaxy syndrome and interrupted inferior caval vein, (c) non-heterotaxy HLHS control group. Boxes represent the median and interquartile ranges (IQR); whiskers represent the 95% confidence interval; individual values represent values outside the 95% confidence interval. HLHS = hypoplastic left heart syndrome, ns = not statistically significant, SpO2 = peripheral oxygen saturation.
To determine if post-Fontan changes in oxygen saturation were different based on diagnosis of pulmonary arteriovenous malformations or severity of bubble study, we analysed changes in oxygen saturations based on these criteria (Figs 3 and 4). Patients diagnosed with pulmonary arteriovenous malformations pre-Fontan (n = 37) had lower oxygen saturations pre-Fontan compared to those not diagnosed with pulmonary arteriovenous malformations (n = 87) (positive diagnosis: 80.0 (76.0, 82.0)%; no diagnosis: 83.0 (79.0, 85.0)%; p < 0.01), but there was no difference in oxygen saturation at Fontan discharge (positive diagnosis: 89.0 (83.0, 92.0)%; no diagnosis: 89.0 (85.0, 93.0)%; p = 0.08) or any post-Fontan time point (Fig 3a). Within-group analyses for those diagnosed with pulmonary arteriovenous malformations and those not diagnosed showed that both groups increased their saturations from the surgery itself (both with p < 0.01) and after Fontan discharge (positive diagnosis: 3 months p < 0.01, 6 months p < 0.01, 12 months p < 0.01, Fig 3b; no diagnosis: 3 months p = 0.03, 6 months p < 0.01, 12 months p < 0.01, Fig 3c).

Figure 3. Tukey Box and whisker plots showing oxygen saturation changes before and after Fontan palliation based on a documented PAVM diagnosis or no documentation of a PAVM diagnosis before Fontan palliation. (a) Between-group comparison, and (b and c) within-group comparisons are shown for both groups. Boxes represent the median and interquartile ranges (IQR); whiskers represent the 95% confidence interval; individual values represent values outside the 95% confidence interval. ns = not statistically significant, PAVM = pulmonary arteriovenous malformation, SpO2 = peripheral oxygen saturation.

Figure 4. Tukey Box and whisker plots showing oxygen saturation changes before and after Fontan palliation for patients who had a contrast echocardiogram (Bubble study) performed pre-Fontan. (a) Between-group comparison, and (b and c) within-group comparisons are shown for patients with a severely positive Bubble study or mild/moderately positive Bubble study. Boxes represent the median and interquartile ranges (IQR); whiskers represent the 95% confidence interval; individual values represent values outside the 95% confidence interval. ns = not statistically significant, SpO2 = peripheral oxygen saturation.
When analysing by severity of bubble study, those patients with a severely positive bubble study pre-Fontan (n = 15) had similar saturations pre-Fontan as those with a mild or moderately positive bubble study (n = 10) (severely positive: 76.0 (75.0, 81.0)%; mild or moderately positive: 80.0 (77.0, 82.0)%; p = 0.43) but did have lower oxygen saturations at Fontan discharge (severely positive: 85.0 (81.0, 89.0)%; mild or moderately positive: 90.5 (85.0, 93.0)%; p = 0.02) (Fig 4a). There was no difference between the two groups at subsequent follow-up at 3, 6, and 12 months post-Fontan. Within-group analyses showed that patients with severely positive bubble studies increased their saturations from the surgery itself (p < 0.01) and after Fontan discharge (3 months p < 0.01, 6 months p < 0.01, 12 months p < 0.01) (Fig 4b). Patients with mild or moderately positive bubble studies increased their saturations from the surgery itself (p < 0.01) but did not increase their saturations after Fontan discharge (3 months p = 0.96, 6 months p = 0.96, 12 months p = 0.56) (Fig 4c), though this sub-analysis is likely underpowered.
Discussion
In this study, patients with heterotaxy syndrome and non-heterotaxy hypoplastic left heart syndrome had variable diagnostic rates of pulmonary arteriovenous malformations pre-Fontan, yet all study groups had increases in oxygen saturation after Fontan discharge. Based on previous studies that attribute increases in oxygen saturation after Fontan discharge to pulmonary microvascular remodeling and resolution of pulmonary arteriovenous malformations, our data indicate that pulmonary arteriovenous malformations are present prior to Fontan palliation and resolve after Fontan palliation for patients with and without heterotaxy syndrome. The specific patient factors that increase susceptibility to pulmonary arteriovenous malformations remain unknown.
In this single-institution retrospective study, pulmonary arteriovenous malformations were diagnosed in the medical chart more frequently in patients with heterotaxy syndrome and interrupted inferior caval vein (Table 2); however, there was not a systematic assessment of pulmonary arteriovenous malformations pre-Fontan. Of those patients who had a contrast echocardiogram (“bubble study”) performed pre-Fontan, all patients had a positive bubble study in at least one lung (25/25) and there was no difference among the groups in the prevalence of severely positive bubble studies, albeit with a small number of patients in each group. Using a quantitative assessment of intrapulmonary shunting with a radionuclide scan, Vettukattil et al previously reported that intrapulmonary right to left shunting (i.e., pulmonary arteriovenous malformations) develops in all patients after superior cavopulmonary connection. Reference Vettukattil, Slavik and Lamb3 Interestingly, in this study of 17 patients with single ventricle CHD and five controls, there were no patients with hypoplastic left heart syndrome. What degree of intrapulmonary shunting (by radionuclide scan or contrast echocardiogram) warrants a diagnosis of pulmonary arteriovenous malformations or correlates with clinical changes in oxygen saturation remains unclear.
Despite a lack of consensus on the prevalence and optimal diagnostic criteria for pulmonary arteriovenous malformations, studies have repeatedly used increased oxygen saturations after Fontan discharge as a surrogate for resolution of pulmonary arteriovenous malformations. Reference Shah, Rychik, Fogel, Murphy and Jacobs4–Reference Alsoufi, Rosenblum and Travers9 Based on this, our study is the first to demonstrate similar increases in oxygen saturation for patients with heterotaxy syndrome and hypoplastic left heart syndrome after Fontan discharge. Similar to previous studies, our data support that clinicians should expect saturations to increase most during the first 3–6 months Reference Shah, Rychik, Fogel, Murphy and Jacobs4,Reference Vollebregt, Pushparajah and Rizvi8 but saturations continue to increase throughout the first year following Fontan palliation. Reference McElhinney, Kreutzer, Lang, Mayer, del Nido and Lock6
Sub-analysis of changes in oxygen saturation based on diagnosis of pulmonary arteriovenous malformations showed that both groups (diagnosed and not diagnosed with pulmonary arteriovenous malformations) increased oxygen saturations after Fontan discharge (Fig 3), which indicates either that pulmonary arteriovenous malformations were incorrectly diagnosed (false positive or false negative) or that post-Fontan increases in oxygen saturation do not accurately reflect resolution of pulmonary arteriovenous malformations. Conversely, sub-analysis also showed that those patients with severely positive bubble studies had increased oxygen saturation after Fontan discharge but those with mildly or moderately positive bubble studies did not (Fig 4). Previous studies report that contrast echocardiography is highly sensitive and can detect pulmonary microvascular disease prior to pulmonary vein desaturation. Reference Feinstein, Moore, Rosenthal, Puchalski and Brook10,Reference Asada, Morishita, Kawai, Kajiyama and Ikeda11 Thus, taken all altogether, we speculate that without systematically screening patients with single ventricle CHD, we are prone to miss diagnoses of pulmonary arteriovenous malformations, and severely positive bubble studies likely identify those patients with more clinically significant pulmonary arteriovenous malformations.
An important consideration for monitoring oxygen saturations post-Fontan, which has not been addressed in the previous studies, is the potential confounding effect of newly developed aortopulmonary collaterals. In this retrospective study, we cannot control for or rule-out that aortopulmonary collaterals developed post-Fontan and increased oxygen saturations; however, Grosse-Wortmann et al report that aortopulmonary collaterals appear to regress spontaneously after Fontan palliation. Reference Grosse-Wortmann, Al-Otay and Yoo12 There are no studies, to our knowledge, that longitudinally assess aortopulmonary collateral formation and/or regression in the immediate post-Fontan follow-up period, corresponding to the follow-up period of our study.
Our retrospective study has several limitations. As a single-institution study, the relatively small number of patients in each sub-group limits generalisability and detailed sub-analyses. Additionally, lack of systematic testing for pulmonary arteriovenous malformations before and after Fontan palliation, as well as inherent physician variability and biases in the diagnosis of pulmonary arteriovenous malformations, limits the strength of our conclusions. Finally, we cannot unequivocally determine that increases in oxygen saturation after Fontan discharge are due to resolution of pulmonary arteriovenous malformations. In addition to potential contribution of aortopulmonary collaterals, spontaneous gradual closure of surgically created Fontan fenestrations could also increase oxygen saturations independent of pulmonary arteriovenous malformation. Unfortunately, no quantitative flow studies were performed to objectively quantify volume of shunting through patent fenestrations.
In conclusion, our data indicate that pulmonary arteriovenous malformations are variably diagnosed prior to Fontan palliation. It remains unclear, though, if there are true differences in susceptibility to pulmonary arteriovenous malformations because patients with and without heterotaxy syndrome have increases in oxygen saturations throughout the first year after Fontan discharge. A quantitative, systematic approach to diagnosis and follow-up pulmonary arteriovenous malformations is needed to better understand pulmonary microvascular remodeling in single ventricle CHD.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
This study was approved by the local Institutional Review Board.