Abnormal small fistulous flows in the pulmonary artery can be detected on routine transthoracic echocardiographic examinations in asymptomatic patients by colour Doppler echocardiography. The differential diagnosis of abnormal fistulous flows in the pulmonary artery includes persistent ductus arteriosus, coronary artery-pulmonary artery fistulas, aortopulmonary windows, and systemic arteriovenous fistulas.Reference Gowda, Vasavada and Khan 1 The most likely diagnosis is small coronary artery-pulmonary artery fistulas. However, it is only possible to reveal the definite aetiology by using cardiac catheterisation. In such cases, the use of an invasive diagnostic method to determine the source of the fistulous flow that is too small to require an intervention is controversial. It is still a dilemma to manage asymptomatic patients with incidentally detected abnormal fistulous flows by colour Doppler in the pulmonary artery. Here, we describe the characteristics of abnormal fistulous flows in the pulmonary artery that were detected on routine echocardiographic examination in 101 patients who had long-term follow-up results.
Materials and methods
The study population consisted of 101 patients with abnormal fistulous flow in the pulmonary artery who had been studied at our institution and Dr Saltık’s private practice between 2005 and 2018. Transthoracic echocardiography was performed with an appropriate transducer interfaced with a GE Vivid 3 and 7 ultrasonography system (GE Healthcare, Wauwatosa, Wisconsin, United States of America) and a Philips IE 33 US system (Philips Healthcare, Andover, Massachusetts, United States of America). Two-dimensional, M-mode, colour-flow Doppler, pulsed Doppler, and continuous-wave Doppler echocardiography were used on all patients. All echocardiographic evaluations were performed in accordance with American Society of Echocardiography guidelines.Reference Lai, Geva and Shirali 2 Patients with abnormal fistulous flow into the pulmonary artery on echocardiographic examination were included in the study. In all patients, flow in the pulmonary artery was incidentally detected during echocardiographic examination and patients did not have any signs or symptoms related to a fistula. If there was no additional pathology, echocardiography was performed annually. The patients’ first admission time (age), time of diagnosis (age), gender, weight at diagnosis, reason for the echo, additional cardiac and non-cardiac pathologies, additional cardiac examinations, follow-up times, and changes in fistula flow at follow-up were evaluated.
The patients were divided into 3 groups according to the localisation of the fistulous flows in the pulmonary artery. Group 1 included those with fistulous flow located in the anterior aspect of the main pulmonary artery (between the pulmonary valve and the bifurcation) (Fig 1a) (Supplementary video S1a); Group 2 included those with fistulous flow located in the aortic side of the main pulmonary artery (Fig 1b) (Supplementary video S1b); Group 3 included those with fistulous flow located in the right pulmonary artery (Fig 1c) (Supplementary video S1c). Statistical analyses were performed using SPSS 15 (SPSS, Chicago, İllinois, United States of America). Patients with no additional cardiac pathology other than fistulous flow were followed up by echocardiographic examination once a year, and the other patients with concomitant cardiac anomalies followed up at different frequencies according to their cardiac pathologies.
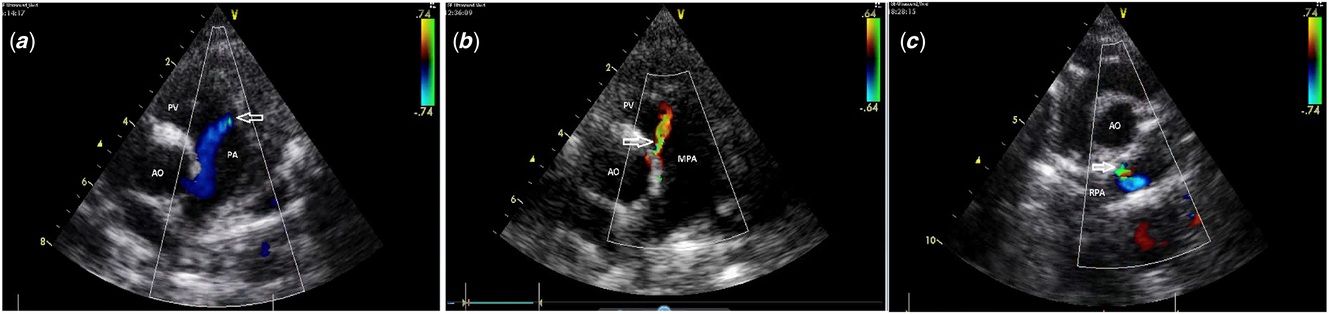
Figure 1. (a) Group 1, fistulous flow located in anterior aspect of main pulmonary artery (between pulmonary valve and bifurcation); (b) Group 2, fistulous flow located in aortic side of main pulmonary artery; and (c) Group 3, fistulous flow located in right pulmonary artery. Arrow shows the opening of the fistula. pv: pulmonary valve; mpa: main pulmonary artery; Ao: aorta; RPA: right pulmonary artery; pa: pulmonary artery.
Results
There were 64 (63.3%) male patients and 37 (36.7%) female patients. Echocardiography was performed mean 4.5 ± 3.1 times (range 2–15) for each patient. The mean age at first echocardiographic evaluation was 4.3 ± 4.2 years (range 1 day to 16 years), but the mean age at diagnosis was 5.28 ± 4.23 years (range 1 day to 16 years). In 79 (78.2%) patients, the fistulous flow in the pulmonary artery was diagnosed at the first presentation, whilst in the remaining 22 patients (21.8%), the fistulous flow was diagnosed after the first examination. In 11 of those patients, fistulous flow was detected in the second, 5 patients in the third, 1 patient in the fourth, 1 patient in the fifth, 2 patients in the seventh, and the remaining 2 patients in the tenth echocardiographic examinations. Of the 22 patients diagnosed late, 19 (86%) had additional cardiac pathology. The weight of the patients at diagnosis was 21.3 ± 14.3 (range 3.5 to 67 kg). The echocardiography indication was cardiac murmur in 42 (41.6%), routine cardiac control in 30 (29.7%), additional CHD in 14 (13.8%), non-specific chest pain in 11 (10.9%), suspicion of inflammatory heart disease in 2 (2%), and syncope in 2 (2%) patients. One patient was previously misdiagnosed in the newborn period as having an abnormal origin of the left coronary artery from the pulmonary artery. Coronary arteries were evaluated as normal, except in seven patients with mild left main coronary artery dilatation on echocardiography. Additional cardiac anomalies were ventricular septal defect in 8, patent ductus arteriosus in 6, atrial septal defect in 5, mitral valve prolapse in 4, coarctation of aorta in 4, bicuspid aortic valve in 3, and Kawasaki disease in 1 patient. One patient had Down syndrome. These fistulous flows were detected in three different regions in the parasternal short axis view. In 70 (69.3%) patients, fistulous flow was located in the anterior aspect of the main pulmonary artery (between the pulmonary valve and the bifurcation), in 23 (22.8%) patients on the aortic side of the main pulmonary artery and in 8 (7.9%) patients in the right pulmonary artery (Fig 2). Cardiac catheterisation was performed in only 3 of the 101 patients for different reasons without any indications from us. A small coronary artery-pulmonary artery fistula was diagnosed with selective coronary angiography in these three patients (Fig 3). An exercise test was performed in only eight patients for different reasons, and no abnormalities were observed. Thirty-seven patients (36.7%) were seen once and were not followed up again. The remaining 64 patients (63.3%) were followed up for a mean of 52.6 ± 43.7 months (range 10 days to 12.7 years). Spontaneous closure was detected in only three patients; the others remained almost unchanged during the follow-up.

Figure 2. Distribution of fistulas according to the opening side on parasternal aortic short axis.

Figure 3. Selective coronary angiography demonstrates the left coronary artery to pulmonary.
Discussion
Echocardiography is a non-invasive and safe method commonly used in the diagnosis of cardiac morphology and CHD.Reference Lai, Geva and Shirali2 While cardiac morphology is recognised by 2D echocardiography, continuous wave Doppler and colour Doppler echocardiography are used to determine cardiac hemodynamic abnormalities. Colour Doppler echocardiography enables the identification of many clinically significant cardiac pathologies, as well as many insignificant cardiac morphological and hemodynamic problems. These include mild valve insufficiency that allows us to diagnose silent carditis,Reference Pastore, De Cunto, Benettoni, Berton, Taddio and Lepore3 determination of the presence of a patent foramen ovale,Reference Holmes, Cohen, Katz and Reeder4 detection of small muscular ventricular septal defects,Reference Chang, Jien, Chen and Hsieh5 and silent ducts.Reference Chehab, Saliba and El-Rassi6 Small fistulous flow in the heart cavities and vessels is one of the subclinical pathologies diagnosed by colour Doppler. Incidentally detected fistula flows in pulmonary artery that are the subject of this article are revealed by the sensitivity and high-tech nature of colour Doppler. In our study, the small fistulous flows in the pulmonary artery were incidentally recognised in most patients by echocardiographic examination performed in the absence of clinical signs and symptoms. Symptoms and signs were irrelevant in 82.2% of the patients (innocent murmur, heart control, and non-specific chest pain). In 13.8% of the patients, the small fistula flow in the pulmonary artery was detected during the evaluation of concomitant cardiac pathology and was not the cause of the clinical findings.
When the diagnosis of an abnormal fistula flow is made, it is important to determine the source and the amount of the shunt. In our patients, the fistulas were very thin and did not cause a hemodynamic change. Although echocardiography (2D and colour Doppler) is very sensitive in detecting the presence of flow, it may not be sufficient to determine its source. Detection of the fistula flow from the source vessel or the expansion of the cardiac chamber gives an idea about the fistula source. In our study, coronary arteries of the patients were evaluated by echocardiography and only seven patients had very mild enlargement in the left main coronary artery. However, no dilatation or abnormal additional vessels were observed in the branches. Since very thin fistulas do not cause an echocardiographic change, this finding has not been considered diagnostic. Detection of the current source of the fistulas is only possible with invasive investigations such as angiography, CT, or MRI. However, the fistulas in our study were too thin to require intervention. The patients were asymptomatic and had no clinical findings; therefore, we did not perform any additional invasive examination in our patients, other than three patients that we preferred to follow-up. Although the sources of the fistulas have not been determined, we consider that these fistulas are thin coronary-pulmonary artery fistulas, especially due to the proximity of the pulmonary artery-coronary arteries and similarities in some previously published reports.Reference Sherwood, Rockenmacher, Colan and Geva7 – Reference Seol, Seo and Song 9 The detection of a small coronary-pulmonary artery fistula in three patients, who underwent cardiac catheterisation, also supports this idea.
In our study, the fistula flow in the pulmonary artery was diagnosed in 78.2% of the patients at the initial examination. In the remaining patients, the fistula flow was noticed in subsequent examinations. Late diagnosis was prolonged to the 10th examination in two patients. We think there are two main reasons for late diagnosis. Echocardiography is a cross-sectional examination, and 3D formations are evaluated by taking sections. If the section of the pulmonary artery does not pass through the fistula region, very thin fistula flow may not be detected. Another possibility is that in patients with additional cardiac pathology, the echocardiographer focused on the main cardiac pathology so that the small fistula flow in the pulmonary artery was overlooked. Of the 22 patients who had late diagnoses, only 3 had normal echocardiographic findings and the others had additional cardiac pathology. This result supports our opinion. Both of the patients diagnosed in the 10th echocardiographic examination had aortic coarctation and were followed up during the post-operative period. Another possibility for late diagnosis is the possibility of an acquired fistula, but there was no pathology to cause acquired coronary fistula in our late-diagnosed patient group except for one with Kawasaki disease.Reference Challoumas, Pericleous, Dimitrakaki, Danelatos and Dimitrakakis10
Since we believe that the source of the incidentally detected small fistula flows in the pulmonary artery by colour Doppler is coronary-pulmonary artery fistulas, our approach has been in this direction. There is general agreement about whether symptomatic patients with a coronary artery fistula should be treated.Reference Sherwood, Rockenmacher, Colan and Geva7–Reference Challoumas, Pericleous, Dimitrakaki, Danelatos and Dimitrakakis10, Reference Buccheri, Chirco, Geraci, Caramanno and Cortese11 According to the American College of Cardiology/American Heart Association guidelines, percutaneous or surgical closure is a Class I recommendation for large fistulae regardless of symptoms and for small- to moderate-sized fistulae with evidence of myocardial ischemia, arrhythmia, ventricular dysfunction, ventricular enlargement, or endarteritis in adults.Reference Warnes, Williams and Bashore12 In asymptomatic patients with high-flow shunting, the indications for closure are to prevent the occurrence of symptoms or complications, especially in the pediatric population.Reference Buccheri, Chirco, Geraci, Caramanno and Cortese11, Reference Balanescu, Sangiorgi, Castelvecchio, Medda and Inglese13 Trivial to small (not clinically discernable), asymptomatic fistulas do not require a specific procedure for closure simply because they are detected incidentally.Reference Sherwood, Rockenmacher, Colan and Geva7, Reference Latson14, Reference Said, Lam and van der Werf15 In this study, all of our patients were asymptomatic, all of the shunts were trivial to small, and all of the fistulas were detected incidentally, so we prefer to follow these patients without further intervention. We did not find any signs and symptoms associated with fistulas during the follow-up period with echocardiography up to 12.7 years (mean: 4.4 years). Our number of patients and our follow-up period indicate that it is an appropriate approach to follow up these patients without additional diagnostic and/or therapeutic intervention.
Spontaneous thrombosis of a coronary artery fistula with secondary closure has been reported mostly for small fistulae in infants younger than 2 years. In these cases, continuous follow-up is of paramount importance.Reference Lai, Geva and Shirali2, Reference Chen, Lin, Weng, Wu, Chien and Huang8, Reference Latson14 Spontaneous closure has been estimated in a review published by Said et al in the pediatric population and adult population to be 8.5 and 3%, respectively.Reference Said, Lam and van der Werf15 They postulated that as most patients are asymptomatic, the continuous murmur is not always detected in the pediatric subjects, consequently cases of spontaneous closure may remain undetected, and the reported 1% rate in the literature is probably an underestimate. In one of the highest spontaneous closure rates published on echocardiographic follow-ups, a multicentre pediatric series of 42 cases were evaluated for asymptomatic heart murmur and the spontaneous closure rate was 17%.Reference Schleich, Rey, Gewillig and Bozio16 Lo et alReference Said, Lam and van der Werf15 followed 122 patients with coronary artery fistula during a mean of 42.58 ± 3.4 months, and the rate of spontaneous closure was 30% in the congenital group and 40% in the asymptomatic group. In our study, 3 of the 64 patients who were followed up had fistula closure and the spontaneous closure rate was 4.6%. Although our patients were asymptomatic and the detected shunts by colour Doppler were trivial to small, we did not observe spontaneous closure in published rates.
An interesting and yet unambiguous finding in our study was the localisation of fistula flows. The patients were divided into three groups according to the localisation of the fistula flows in the pulmonary artery (Fig 2). Group 1, where fistula flow was located in the anterior aspect of the main pulmonary artery (between the pulmonary valve and the bifurcation), was the largest. Two-thirds of our patients were in this group. In the literature review, we could not find a study showing the distribution of fistulas in the pulmonary artery according to the opening sites as we did. The type of fistulas described in the case reports was group 1 according to our study.Reference Sherwood, Rockenmacher, Colan and Geva7–Reference Seol, Seo and Song9, Reference Buccheri, Chirco, Geraci, Caramanno and Cortese11 In our study, the opening location of the fistula in three patients who were definitively diagnosed with angiography was also in the group 1 region. In the second group, the fistula was on the aortic side of the pulmonary artery at the parasternal short axis view. One-fifth of the patients were in this group. There were no reports that describe fistulas between the aortic side and pulmonary artery in the current literature. We believe that these flows are due to vasculogenesis abnormalities due to small, short fistulous vessels associated with the left main coronary artery. Less than 10% of the small fistula flows detected in echocardiographic examinations was within the right pulmonary artery. In the literature, a limited number of coronary artery fistulas in the right pulmonary artery have been described.Reference Wayangankar, Payne, Po and Sivaram17, Reference DeBakey and Lawrie18 In the study that presented the long-term follow-up results of 122 patients, there was fistula flow within the right pulmonary artery in 9 patients.Reference Lo, Lin and Hsieh19 In our study, we also believe that the small flows in the right pulmonary artery originate from coronary arteries. On the other hand, because the right pulmonary artery region is located more distally to the coronary arteries, the source of fistulae flows may be the systemic arteries.Reference Ruberti, Odero and Arpesani20, Reference Akahane, Kurokawa, Takahashi, Satomi and Sasaki21 Although both patients with the coronary artery-right pulmonary artery and systemic artery-right pulmonary artery fistulas have been described,Reference Osada and Nakajima22 the systemic artery-pulmonary artery fistula is much rarer. Published patients with systemic artery-pulmonary artery fistulas are usually adult and symptomatic patients. In our study, we could not identify a patient with fistulous flow in the left pulmonary artery. There is only 1 patient in the series of 122 patients published by Lo et al.Reference Lo, Lin and Hsieh19 One of the possible reasons for our lack of thin fistula flow in the left pulmonary artery is that the imaging of the left pulmonary artery with echocardiography is not as easy or clear as with the right pulmonary artery.
Since we believe that the source of fistula flows which were detected in pulmonary artery is coronary fistulas, we also evaluated the additional cardiac anomalies in our patients. There were different cardiac anomalies in the published coronary fistula series. In the study reported by Holzer et al,Reference Holzer, Johnson, Ciotti, Pozzi and Kitchiner 23 5 of the 17 (29%) patients had additional cardiac anomalies. They also reviewed coronary fistulas reported in children in the English literature and found additional cardiac anomalies in 106 (25%) of 426 patients. The most commonly associated anomaly was the spectrum of tetralogy of Fallot, including tetralogy with pulmonary atresia. The next most common anomalies were atrial septal defects and ventricular septal defects. Said et al reviewed the English literature describing coronary artery fistulas between 1993 and August, 2004 and found that the incidence of associated congenital cardiac anomalies was 13% in adults and 21% in pediatric populations and ranged from 5 to 30%.Reference Said, Lam and van der Werf 15 The highest associated congenital cardiac anomaly incidence in the pediatric series reported by Wong and Menahem was 38%.Reference Wong and Menahem 24 In our study, 29.7% (30 patients) of patients had additional cardiac anomalies. The most commonly detected congenital cardiac anomalies in our study were ventricular septal defects, patent ductus arteriosus, and atrial septal defects, which constituted two-thirds of the patients. There were no patients with tetralogy of Fallot in our study as reported by Holzer et al, and the rates of additional anomalies and in our study were consistent with the results of Sherwood MC et al.Reference Sherwood, Rockenmacher, Colan and Geva 7 , Reference Holzer, Johnson, Ciotti, Pozzi and Kitchiner 23
We consider that fistula flow may be acquired in only one of our patients. In this 4-year-old patient, the diagnosis was made concurrently with the diagnosis of Kawasaki disease and no subsequent closure was observed in follow-up. Acquired coronary artery fistulas associated with Kawasaki disease have been described previously. In the series reported by Liang et al,Reference Liang, Kuo, Yang, Wang and Ko 25 17 of the 325 patients with Kawasaki disease had coronary fistula. Lo et al presented 22 patients with coronary fistula related to Kawasaki disease and 1 patient related to previously performed open-heart surgery in 122 patients with coronary fistula.Reference Lo, Lin and Hsieh 19 In a study by Liang et al, 14 patients had fistula openings in the main pulmonary artery and 3 in the right pulmonary artery.Reference Liang, Kuo, Yang, Wang and Ko 25 In the series of Lo et al, 82% (18 patients) of the Kawasaki-related acquired fistulas were within the main pulmonary artery.Reference Lo, Lin and Hsieh 19 The ratio of Kawasaki disease is low in our patients; however, it is similar to the current literature proportionally.
Conclusion
Abnormal small fistula flows in the pulmonary artery can be detected on routine echocardiographic examination in asymptomatic patients by colour Doppler echocardiography. The most likely diagnosis is a small coronary artery-pulmonary artery fistula. During mid- to long-term follow-up, these patients do not have a cardiac problem and a low rate of spontaneous fistula closure. Since the fistulas are thin and hemodynamically insignificant, it is appropriate to monitor these patients without performing any invasive diagnostic procedures for the fistula source.
Acknowledgements
None.
Financial Support
The clinicians received no grants from any funding agency and no support from any commercial or not-for-profit sector.
Conflicts of Interest
None.
Ethical Standards
The authors assert that this work complies with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. This case was approved by the patients family.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951119002051





